Biology:Morula
| Morula | |
|---|---|
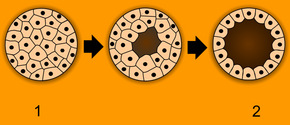 Blastulation. 1 - morula, 2 - blastula. | |
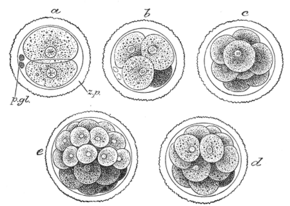 First stages of segmentation of a fertilized mammalian ovum. Semidiagrammatic. z.p. Zona pellucida. p.gl. Polar bodies. a. Two-cell stage. b. Four-cell stage. c. Eight-cell stage. d, e. Morula stage. | |
| Details | |
| Days | 3 |
| Precursor | Zygote |
| Gives rise to | Blastula, Blastocyst |
| Anatomical terminology | |
A morula (Latin, morus: mulberry) is an early-stage embryo consisting of a solid ball of cells called blastomeres, contained in mammals, and other animals within the zona pellucida shell. The blastomeres are the daughter cells of the zygote, and when the blastomeres number from 16–32 the ball of cells is called a morula.[1][2] At the 8-cell stage the blastomeres are round and only loosely adhered. With further division they begin to become flattened, and develop an inside-out polarity that optimises the cell to cell contact.[1]
In the human embryo by day four after fertilisation, the morula at about the 32–cell stage begins to take in fluid. The fluid comes about from sodium-potassium pumps (on trophoblasts) that pump sodium into the morula, drawing in water from the maternal environment to become blastocoelic fluid. Hydrostatic pressure of the fluid creates a large cavity in the morula called a blastocoel or blastocyst cavity. Embryoblast cells also known as the inner cell mass form a compact mass of cells at the embryonic pole on one side of the cavity. The trophoblast is organized into a thin sheet of tightly adhered epithelial cells. The embryo is now termed a blastocyst.[1]
Formation
The morula is produced by a series of cleavage divisions of the early embryo, starting with the single-celled zygote. Once the embryo has divided into 16 cells, it begins to resemble a mulberry, hence the name morula (Latin, morus: mulberry).[3] Within a few days after fertilization, cells on the outer part of the morula become bound tightly together with the formation of desmosomes and gap junctions, becoming nearly indistinguishable. This process is known as compaction.[4][5] The cells on the outside and inside become differentially fated into trophoblast (outside) and inner cell mass (inside) progenitors. A cavity forms inside the morula, by the active transport of sodium ions from trophoblast cells and osmosis of water. This results in a hollow ball of cells known as the blastocyst in placental mammals, and the blastula in other animals.[6][7] The blastocyst's outer cells will become the first embryonic epithelium (the trophectoderm). Some cells, however, will remain trapped in the interior and will become the inner cell mass (ICM), and are pluripotent. In mammals (except monotremes), the ICM will ultimately form the "embryo proper", while the trophectoderm will form the placenta and extra-embryonic tissues. However, reptiles have a different ICM. The stages are prolonged and divided in four parts.[8][9][10][11]
References
- ↑ 1.0 1.1 1.2 Schoenwolf, Gary C. (2015). Larsen's human embryology (Fifth ed.). Philadelphia, PA. pp. 35–37. ISBN 9781455706846.
- ↑ Boklage, Charles E. (2009). How New Humans Are Made: Cells and Embryos, Twins and Chimeras, Left and Right, Mind/Self/Soul, Sex, and Schizophrenia. World Scientific. p. 217. ISBN 9789812835130. https://books.google.com/books?id=j7GfgRIgP9YC&pg=PT233.
- ↑ Human embryology (3rd ed.). Elsevier Health Sciences. 2001. p. 20. ISBN 978-0-443-06583-5. https://books.google.com/books?id=H5qw-jiMc44C&pg=PA20.
- ↑ Chard, Tim & Lilford, Richard (1995). Basic sciences for obstetrics and gynaecology. Springer. p. 18. ISBN 978-3-540-19903-8. https://books.google.com/books?id=MsPsw4ejMxwC&pg=PA18.
- ↑ Mercader, Amparo (2008). "Human embryo culture". Essential stem cell methods. Academic Press. p. 343. ISBN 978-0-12-374741-9. https://books.google.com/books?id=aPcvphPV6AgC&pg=PA343.
- ↑ Patestas, Maria Antoniou; Gartner, Leslie P. (2006). A textbook of neuroanatomy. Wiley-Blackwell. p. 11. ISBN 978-1-4051-0340-4. https://books.google.com/books?id=JEEnkNuuYkoC&pg=PA11.
- ↑ Geisert, R.D.; Malayer, J.R. (2000). "Implantation: Blastocyst formation". Reproduction in farm animals. Wiley-Blackwell. p. 118. ISBN 978-0-683-30577-7. https://books.google.com/books?id=baz2TC1y2esC&pg=PA118.
- ↑ Morali, Olivier G. (2005). "Epithelium-Mesenchyme Transitions are Crucial Morphogenetic Events Occurring During Early Development". in Savagner, Pierre. Rise and fall of epithelial phenotype: concepts of epithelial-mesenchymal transition. Springer. p. 16. ISBN 978-0-306-48239-7. https://books.google.com/books?id=3a54uGVK03cC&pg=PA16.
- ↑ Birchmeier, Carmen (1997). "Morphogenesis of epithelial cells". Adhesion molecules in health and disease. CRC Press. p. 208. ISBN 978-0-8247-9824-6. https://books.google.com/books?id=vLqxqS0iZkYC&pg=PA208.
- ↑ Nagy, András (2003). Manipulating the mouse embryo: a laboratory manual. CSHL Press. pp. 60–61. ISBN 978-0-87969-591-0. https://books.google.com/books?id=4juoa5xMs8oC&pg=PA60.
- ↑ Connell, R.J.; Cutner, A. (2001). "Basic Embryology". Textbook of female urology and urogynaecology. Taylor & Francis. p. 92. ISBN 978-1-901865-05-9. https://books.google.com/books?id=EMMUyiKOOiEC&pg=PA92.
Further reading
