Biology:Oxygen rebound mechanism
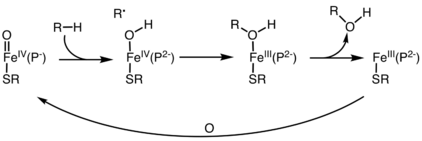
In biochemistry, the oxygen rebound mechanism is the pathway for hydroxylation of organic compounds by iron-containing oxygenases. Many enzymes effect the hydroxylation of hydrocarbons as a means for biosynthesis, detoxification, gene regulation, and other functions. These enzymes often utilize Fe-O centers that convert C-H bonds into C-OH groups. The oxygen rebound mechanism starts with abstraction of H from the hydrocarbon, giving an organic radical and an iron hydroxide. In the rebound step, the organic radical attacks the Fe-OH center to give an alcohol group, which is bound to Fe as a ligand. Dissociation of the alcohol from the metal allows the cycle to start anew. This mechanistic scenario is an alternative to the direct insertion of an O center into a C-H bond. The pathway is an example of C-H activation.[1][2]
Three main classes of these enzymes are cytochrome P450, alpha-ketoglutarate-dependent hydroxylases, and nonheme-diiron hydroxylases.
References
- ↑ Huang, X.; Groves, J. T. (2017). "Beyond ferryl-mediated hydroxylation: 40 years of the rebound mechanism and C–H activation". Journal of Biological Inorganic Chemistry 22 (2–3): 185–207. doi:10.1007/s00775-016-1414-3. PMID 27909920.
- ↑ Oxygenation reactions by high-valent iron-oxo species are "C–H activation" reactions in the broad sense of the term. Some authors would call this a C–H functionalization but not an "activation" (in its narrow sense), since a metal–carbon bond is not involved in the C–H bond cleaving process.
 |