Biology:Photochemical action plots
Photochemical action plots are a scientific tool used to understand the effects of different wavelengths of light on photochemical reactions. The methodology involves exposing a reaction solution to the same number of photons at varying monochromatic wavelengths, monitoring the conversion or reaction yield of starting materials and/or reaction products. Such global high-resolution analysis of wavelength-dependent chemical reactivity has revealed that maxima in absorbance and reactivity often do not align.[1] Photochemical action plots are historically connected to (biological) action spectra.
Historical Development
The study of biological responses to specific wavelengths dates back to the late 19th century. Research primarily focused on assessing photodamage from solar radiation using broad-band lamps and narrow filters. These studies quantified effects such as cell viability,[2] production of erythema,[3] vitamin D3 degradation,[4][5] DNA changes,[6][7] and skin cancer appearance.[8] The first biological action spectrum was recorded by Engelmann, who used a prism to produce different colors of light and then illuminated cladophora in a bacteria suspension. He discovered the effects of different light wavelengths on photosynthesis, marking the first recorded action spectrum of photosynthesis.[9]
Critical evaluations of active wavelength regions in these studies helped identify contributing chromophores to processes such as photosynthesis. These chromophores are key for converting solar energy into chemical energy, with their absorption closely matching the rate of photosynthesis, usually determined by oxygen production or carbon fixation.[10] This correlation led to the discovery of chlorophyll as a key chromophore in plant growth. Such studies have also been instrumental in identifying DNA as the core genetic material,[11] key wavelengths leading to skin cancer,[12] the transparent optical window of biological tissue,[13] and the influence of color on circadian rhythms.[14]
In the late 20th century, action spectra became essential in developing optical devices for photocatalysis[15] and photovoltaics,[16] particularly in measuring photocurrent efficiency at various wavelengths. These studies have been vital in understanding primary contributors to photocurrent generation,[17][18] leading to advancements in materials,[19][20] morphologies,[21][22] and device designs[23][24] for improved solar energy capture and utilization.
In photochemistry, action spectra have been mainly used in photodissociation studies. These involve a monochromatic light source, often a laser, coupled with a mass spectrometer to record wavelength-dependent ion dissociation in gaseous phases.[25] These spectra help identify contributing chromophores in molecular systems,[26][27] characterize radical generation and unstable isomers,[28][29] and understand higher state electron dynamics.[30][31]
The field underwent a transformation when a team led by Barner-Kowollik and Gescheidt recorded the first modern-day photochemical action plot using a tuneable monochromatic nanosecond pulsed laser system, discovering a strong mismatch between photochemical reactivity and absorptivity and marking a critical advancement in mapping wavelength-dependent conversions in photoinduced polymerizations.[32] Following this, numerous photochemical action plots have been recorded in various molecular and polymerization systems.[33][34]
Experimental Setup
Key differences between traditional (biological) action spectra and modern photochemical action plots lie in the precision resolution of wavelengths (monochromaticity) and that an exact number of photons at each wavelength is applied coupled with the fact that covalent bond forming reactions were investigated for the first time.[32]
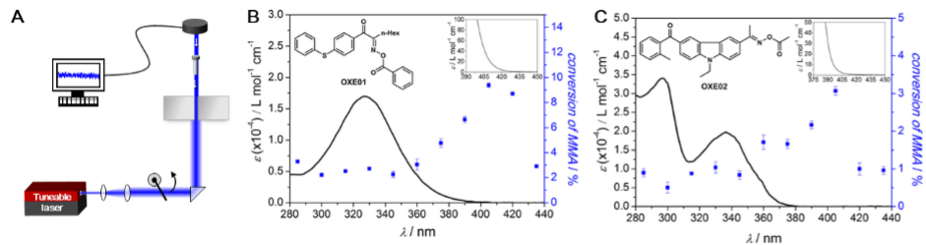
In the field of photochemical analysis, it is common to measure the extinction of chemicals with high precision, often at the sub-nanometer scale, using UV/Vis spectroscopy. To understand fundamental relationships between a chemical's absorbance and its photoreactivity, a detailed analysis of the reactivity at a similar level of resolution is required. Traditional methods using broadly emitting light sources or filters have inherent limitations in resolving true wavelength dependence in photoreactivity. To record an action plot, a wavelength-tuneable laser system is employed, capable of delivering a stable number of photons at each wavelength. The photoreactive reaction mixture is divided into aliquots and subjected to monochromatic light independently. The photochemical process' yield or conversion is subsequently measured using sensors like UV-Vis absorption or nuclear magnetic resonance (NMR) frequency changes.
Findings and Implications
A key finding of modern photochemical action plots[32] is that the absorption spectrum of a photoreactive molecule or reaction mixture correlates poorly with photochemical reactivity as a function of wavelength in many cases. Initial studies showed a significant red-shift in photopolymerization yield compared to the absorption spectrum of the employed photoinitiators, which showed extremely low absorptivity in those regions. This mismatch between absorption spectra and photochemical action plots has by now been observed in a wide array of photoreactive systems.[35][36][37] A prominent example is the photoinduced [2+2] cycloaddition of the stilbene derivative, styrypyrene, which exhibited an 80 nm discrepancy between the action plot and absorption spectrum.[33] Current research focuses on understanding the reasons behind these frequently observed mismatches. For photochemical applications, the consequences of the absorptivity/reactivity mismatch are far reaching, as only photochemical action plots can reveal the most effective wavelength for a given process, moving away from the past paradigm that absorption spectra provide guidance for selecting the most effective wavelength.
References
- ↑ Walden, Sarah L.; Carroll, Joshua A.; Unterreiner, Andreas-Neil; Barner-Kowollik, Christopher (2023-11-08). "Photochemical Action Plots Reveal the Fundamental Mismatch Between Absorptivity and Photochemical Reactivity" (in en). Advanced Science: e2306014. doi:10.1002/advs.202306014. ISSN 2198-3844. PMID 37937391. PMC 10797470. https://onlinelibrary.wiley.com/doi/10.1002/advs.202306014.
- ↑ Neuman, Keir C.; Chadd, Edmund H.; Liou, Grace F.; Bergman, Keren; Block, Steven M. (November 1999). "Characterization of Photodamage to Escherichia coli in Optical Traps" (in en). Biophysical Journal 77 (5): 2856–2863. doi:10.1016/S0006-3495(99)77117-1. PMID 10545383. Bibcode: 1999BpJ....77.2856N.
- ↑ Schmalwieser, Alois W.; Wallisch, Silvia; Diffey, Brian (December 2012). "A library of action spectra for erythema and pigmentation" (in en). Photochemical & Photobiological Sciences 11 (2): 251–268. doi:10.1039/c1pp05271c. ISSN 1474-905X. PMID 22194032. https://link.springer.com/10.1039/c1pp05271c.
- ↑ MacLaughlin, J. A.; Anderson, R. R.; Holick, M. F. (1982-05-28). "Spectral Character of Sunlight Modulates Photosynthesis of Previtamin D 3 and Its Photoisomers in Human Skin" (in en). Science 216 (4549): 1001–1003. doi:10.1126/science.6281884. ISSN 0036-8075. PMID 6281884. https://www.science.org/doi/10.1126/science.6281884.
- ↑ Norval, Mary; Björn, Lars Olof; de Gruijl, Frank R. (January 2010). "Is the action spectrum for the UV-induced production of previtamin D3 in human skin correct?" (in en). Photochemical & Photobiological Sciences 9 (1): 11–17. doi:10.1039/b9pp00012g. ISSN 1474-905X. PMID 20062839. https://link.springer.com/10.1039/b9pp00012g.
- ↑ Setlow, Richard B.; Setlow, Jane K. (June 1972). "Effects of Radiation on Polynucleotides" (in en). Annual Review of Biophysics and Bioengineering 1 (1): 293–346. doi:10.1146/annurev.bb.01.060172.001453. ISSN 0084-6589. PMID 4567755. https://www.annualreviews.org/doi/10.1146/annurev.bb.01.060172.001453.
- ↑ Freeman, S E; Hacham, H; Gange, R W; Maytum, D J; Sutherland, J C; Sutherland, B M (July 1989). "Wavelength dependence of pyrimidine dimer formation in DNA of human skin irradiated in situ with ultraviolet light." (in en). Proceedings of the National Academy of Sciences 86 (14): 5605–5609. doi:10.1073/pnas.86.14.5605. ISSN 0027-8424. PMID 2748607. Bibcode: 1989PNAS...86.5605F.
- ↑ de Gruijl, F. R.; Sterenborg, H. J.; Forbes, P. D.; Davies, R. E.; Cole, C.; Kelfkens, G.; van Weelden, H.; Slaper, H. et al. (1993-01-01). "Wavelength dependence of skin cancer induction by ultraviolet irradiation of albino hairless mice". Cancer Research 53 (1): 53–60. ISSN 0008-5472. PMID 8416751. https://aacrjournals.org/cancerres/article/53/1/53/498742/Wavelength-Dependence-of-Skin-Cancer-Induction-by. Retrieved 2023-12-16.
- ↑ Mcgraw-Hill, Tata (in en). Question Bank In Biology For Class Xi. McGraw-Hill Education (India) Pvt Limited. ISBN 978-0-07-026383-3. https://books.google.com/books?id=yrTt7ufgdtYC&pg=PA311.
- ↑ "XIII. On the action of light upon plants, and of plants upon the atmosphere" (in en). Philosophical Transactions of the Royal Society of London 126: 149–175. 1836-12-31. doi:10.1098/rstl.1836.0015. ISSN 0261-0523. https://royalsocietypublishing.org/doi/10.1098/rstl.1836.0015.
- ↑ Gates, Frederick L. (1930-09-20). "A Study of the Bactericidal Action of Ultra Violet Light" (in en). Journal of General Physiology 14 (1): 31–42. doi:10.1085/jgp.14.1.31. ISSN 1540-7748. PMID 19872573. PMC 2141090. https://rupress.org/jgp/article/14/1/31/11237/A-STUDY-OF-THE-BACTERICIDAL-ACTION-OF-ULTRA-VIOLET.
- ↑ Setlow, R B; Grist, E; Thompson, K; Woodhead, A D (1993-07-15). "Wavelengths effective in induction of malignant melanoma." (in en). Proceedings of the National Academy of Sciences 90 (14): 6666–6670. doi:10.1073/pnas.90.14.6666. ISSN 0027-8424. PMID 8341684. Bibcode: 1993PNAS...90.6666S.
- ↑ Anderson, R. Rox; Parrish, John A. (July 1981). "The Optics of Human Skin" (in en). Journal of Investigative Dermatology 77 (1): 13–19. doi:10.1111/1523-1747.ep12479191. PMID 7252245. https://linkinghub.elsevier.com/retrieve/pii/S0022202X15461251.
- ↑ Brainard, George C.; Hanifin, John P.; Greeson, Jeffrey M.; Byrne, Brenda; Glickman, Gena; Gerner, Edward; Rollag, Mark D. (2001-08-15). "Action Spectrum for Melatonin Regulation in Humans: Evidence for a Novel Circadian Photoreceptor" (in en). The Journal of Neuroscience 21 (16): 6405–6412. doi:10.1523/JNEUROSCI.21-16-06405.2001. ISSN 0270-6474. PMID 11487664.
- ↑ Melchionna, Michele; Fornasiero, Paolo (2020-05-15). "Updates on the Roadmap for Photocatalysis" (in en). ACS Catalysis 10 (10): 5493–5501. doi:10.1021/acscatal.0c01204. ISSN 2155-5435. https://pubs.acs.org/doi/10.1021/acscatal.0c01204.
- ↑ Nayak, Pabitra K.; Mahesh, Suhas; Snaith, Henry J.; Cahen, David (2019-03-28). "Photovoltaic solar cell technologies: analysing the state of the art" (in en). Nature Reviews Materials 4 (4): 269–285. doi:10.1038/s41578-019-0097-0. ISSN 2058-8437. Bibcode: 2019NatRM...4..269N. https://www.nature.com/articles/s41578-019-0097-0.
- ↑ Pettersson, Leif A. A.; Roman, Lucimara S.; Inganäs, Olle (1999-07-01). "Modeling photocurrent action spectra of photovoltaic devices based on organic thin films" (in en). Journal of Applied Physics 86 (1): 487–496. doi:10.1063/1.370757. ISSN 0021-8979. Bibcode: 1999JAP....86..487P. https://pubs.aip.org/jap/article/86/1/487/489720/Modeling-photocurrent-action-spectra-of.
- ↑ Terao, Yuhki; Sasabe, Hiroyuki; Adachi, Chihaya (2007-03-05). "Correlation of hole mobility, exciton diffusion length, and solar cell characteristics in phthalocyanine/fullerene organic solar cells" (in en). Applied Physics Letters 90 (10). doi:10.1063/1.2711525. ISSN 0003-6951. Bibcode: 2007ApPhL..90j3515T. https://pubs.aip.org/apl/article/90/10/103515/333034/Correlation-of-hole-mobility-exciton-diffusion.
- ↑ Cushing, Scott K.; Li, Jiangtian; Meng, Fanke; Senty, Tess R.; Suri, Savan; Zhi, Mingjia; Li, Ming; Bristow, Alan D. et al. (2012-09-12). "Photocatalytic Activity Enhanced by Plasmonic Resonant Energy Transfer from Metal to Semiconductor" (in en). Journal of the American Chemical Society 134 (36): 15033–15041. doi:10.1021/ja305603t. ISSN 0002-7863. PMID 22891916. https://pubs.acs.org/doi/10.1021/ja305603t.
- ↑ Kuang, Daibin; Uchida, Satoshi; Humphry-Baker, Robin; Zakeeruddin, Shaik M.; Grätzel, Michael (2008-02-22). "Organic Dye-Sensitized Ionic Liquid Based Solar Cells: Remarkable Enhancement in Performance through Molecular Design of Indoline Sensitizers" (in en). Angewandte Chemie International Edition 47 (10): 1923–1927. doi:10.1002/anie.200705225. ISSN 1433-7851. PMID 18214873. https://onlinelibrary.wiley.com/doi/10.1002/anie.200705225.
- ↑ Sun, Baoquan; Snaith, Henry J.; Dhoot, Anoop S.; Westenhoff, Sebastian; Greenham, Neil C. (2005-01-01). "Vertically segregated hybrid blends for photovoltaic devices with improved efficiency" (in en). Journal of Applied Physics 97 (1): 014914–014914–6. doi:10.1063/1.1804613. ISSN 0021-8979. Bibcode: 2005JAP....97a4914S. https://pubs.aip.org/jap/article/97/1/014914/896687/Vertically-segregated-hybrid-blends-for.
- ↑ Wang, Zhong-Sheng; Kawauchi, Hiroshi; Kashima, Takeo; Arakawa, Hironori (July 2004). "Significant influence of TiO2 photoelectrode morphology on the energy conversion efficiency of N719 dye-sensitized solar cell" (in en). Coordination Chemistry Reviews 248 (13–14): 1381–1389. doi:10.1016/j.ccr.2004.03.006. https://linkinghub.elsevier.com/retrieve/pii/S0010854504000530.
- ↑ Ghosh, Amal K.; Morel, Don L.; Feng, Tom; Shaw, Robert F.; Rowe, Charles A. (1974-01-01). "Photovoltaic and rectification properties of Al/Mg phthalocyanine/Ag Schottky-barrier cells" (in en). Journal of Applied Physics 45 (1): 230–236. doi:10.1063/1.1662965. ISSN 0021-8979. Bibcode: 1974JAP....45..230G. https://pubs.aip.org/jap/article/45/1/230/171261/Photovoltaic-and-rectification-properties-of-Al-Mg.
- ↑ Thompson, Barry C.; Kim, Young-Gi; Reynolds, John R. (2005-06-01). "Spectral Broadening in MEH-PPV:PCBM-Based Photovoltaic Devices via Blending with a Narrow Band Gap Cyanovinylene−Dioxythiophene Polymer" (in en). Macromolecules 38 (13): 5359–5362. doi:10.1021/ma0505934. ISSN 0024-9297. Bibcode: 2005MaMol..38.5359T. https://pubs.acs.org/doi/10.1021/ma0505934.
- ↑ Dunbar, Robert C.; Teng, Harry Ho I.; Fu, Emil W. (October 1979). "Photodissociation spectroscopy of halogen-substituted benzene ions" (in en). Journal of the American Chemical Society 101 (22): 6506–6510. doi:10.1021/ja00516a004. ISSN 0002-7863. https://pubs.acs.org/doi/abs/10.1021/ja00516a004.
- ↑ Polfer, Nicolas C.; Stedwell, Corey N. (2013), "Infrared Photodissociation of Biomolecular Ions", Lecture Notes in Chemistry (Cham: Springer International Publishing): pp. 71–91, doi:10.1007/978-3-319-01252-0_4, ISBN 978-3-319-01251-3, http://dx.doi.org/10.1007/978-3-319-01252-0_4, retrieved 2023-12-16
- ↑ Uleanya, Kelechi O.; Dessent, Caroline E. H. (2021). "Investigating the mapping of chromophore excitations onto the electron detachment spectrum: photodissociation spectroscopy of iodide ion–thiouracil clusters" (in en). Physical Chemistry Chemical Physics 23 (2): 1021–1030. doi:10.1039/D0CP05920J. ISSN 1463-9076. PMID 33428696. Bibcode: 2021PCCP...23.1021U. http://xlink.rsc.org/?DOI=D0CP05920J.
- ↑ Cabré, Gisela; Garrido-Charles, Aida; Moreno, Miquel; Bosch, Miquel; Porta-de-la-Riva, Montserrat; Krieg, Michael; Gascón-Moya, Marta; Camarero, Núria et al. (2019-02-22). "Rationally designed azobenzene photoswitches for efficient two-photon neuronal excitation" (in en). Nature Communications 10 (1): 907. doi:10.1038/s41467-019-08796-9. ISSN 2041-1723. PMID 30796228. Bibcode: 2019NatCo..10..907C.
- ↑ Marlton, Samuel J. P.; McKinnon, Benjamin I.; Ucur, Boris; Bezzina, James P.; Blanksby, Stephen J.; Trevitt, Adam J. (2020-05-21). "Discrimination between Protonation Isomers of Quinazoline by Ion Mobility and UV-Photodissociation Action Spectroscopy" (in en). The Journal of Physical Chemistry Letters 11 (10): 4226–4231. doi:10.1021/acs.jpclett.0c01009. ISSN 1948-7185. PMID 32368922. https://pubs.acs.org/doi/10.1021/acs.jpclett.0c01009.
- ↑ Wellman, Sydney M. J.; Jockusch, Rebecca A. (2015-06-18). "Moving in on the Action: An Experimental Comparison of Fluorescence Excitation and Photodissociation Action Spectroscopy" (in en). The Journal of Physical Chemistry A 119 (24): 6333–6338. doi:10.1021/acs.jpca.5b04835. ISSN 1089-5639. PMID 26020810. Bibcode: 2015JPCA..119.6333W. https://pubs.acs.org/doi/10.1021/acs.jpca.5b04835.
- ↑ Wellman, Sydney M. J.; Jockusch, Rebecca A. (2017-06-07). "Tuning the Intrinsic Photophysical Properties of Chlorophyll a" (in en). Chemistry – A European Journal 23 (32): 7728–7736. doi:10.1002/chem.201605167. ISSN 0947-6539. PMID 27976433. https://chemistry-europe.onlinelibrary.wiley.com/doi/10.1002/chem.201605167.
- ↑ 32.0 32.1 32.2 Fast, David E.; Lauer, Andrea; Menzel, Jan P.; Kelterer, Anne-Marie; Gescheidt, Georg; Barner-Kowollik, Christopher (2017-03-14). "Wavelength-Dependent Photochemistry of Oxime Ester Photoinitiators" (in en). Macromolecules 50 (5): 1815–1823. doi:10.1021/acs.macromol.7b00089. ISSN 0024-9297. Bibcode: 2017MaMol..50.1815F. https://pubs.acs.org/doi/10.1021/acs.macromol.7b00089.
- ↑ 33.0 33.1 Marschner, David E.; Frisch, Hendrik; Offenloch, Janin T.; Tuten, Bryan T.; Becer, C. Remzi; Walther, Andreas; Goldmann, Anja S.; Tzvetkova, Pavleta et al. (2018-05-22). "Visible Light [2 + 2 Cycloadditions for Reversible Polymer Ligation"] (in en). Macromolecules 51 (10): 3802–3807. doi:10.1021/acs.macromol.8b00613. ISSN 0024-9297. Bibcode: 2018MaMol..51.3802M. https://pubs.acs.org/doi/10.1021/acs.macromol.8b00613.
- ↑ Menzel, Jan P.; Noble, Benjamin B.; Lauer, Andrea; Coote, Michelle L.; Blinco, James P.; Barner-Kowollik, Christopher (2017-11-08). "Wavelength Dependence of Light-Induced Cycloadditions" (in en). Journal of the American Chemical Society 139 (44): 15812–15820. doi:10.1021/jacs.7b08047. ISSN 0002-7863. PMID 29024596. https://pubs.acs.org/doi/10.1021/jacs.7b08047.
- ↑ Ma, Congkai; Han, Ting; Efstathiou, Spyridon; Marathianos, Arkadios; Houck, Hannes A.; Haddleton, David M. (2022-11-22). "Aggregation-Induced Emission Poly(meth)acrylates for Photopatterning via Wavelength-Dependent Visible-Light-Regulated Controlled Radical Polymerization in Batch and Flow Conditions" (in en). Macromolecules 55 (22): 9908–9917. doi:10.1021/acs.macromol.2c01413. ISSN 0024-9297. PMID 36438594. Bibcode: 2022MaMol..55.9908M.
- ↑ Reeves, Jennifer A.; De Alwis Watuthanthrige, Nethmi; Boyer, Cyrille; Konkolewicz, Dominik (November 2019). "Intrinsic and Catalyzed Photochemistry of Phenylvinylketone for Wavelength-Sensitive Controlled Polymerization" (in en). ChemPhotoChem 3 (11): 1171–1179. doi:10.1002/cptc.201900052. ISSN 2367-0932. https://chemistry-europe.onlinelibrary.wiley.com/doi/10.1002/cptc.201900052.
- ↑ Irshadeen, Ishrath Mohamed; Walden, Sarah L.; Wegener, Martin; Truong, Vinh X.; Frisch, Hendrik; Blinco, James P.; Barner-Kowollik, Christopher (2021-12-22). "Action Plots in Action: In-Depth Insights into Photochemical Reactivity" (in en). Journal of the American Chemical Society 143 (50): 21113–21126. doi:10.1021/jacs.1c09419. ISSN 0002-7863. PMID 34859671. https://pubs.acs.org/doi/10.1021/jacs.1c09419.
 |

