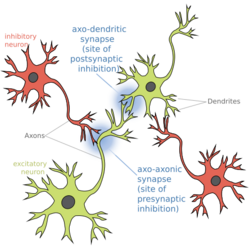
Biology:Presynaptic inhibition
Presynaptic inhibition is a phenomenon in which an inhibitory neuron provides synaptic input to the axon of another neuron (axo-axonal synapse) to make it less likely to fire an action potential. Presynaptic inhibition occurs when an inhibitory neurotransmitter, like GABA, acts on GABA receptors on the axon terminal. Or when endocannabinoids act as retrograde messengers by binding to presynaptic CB1 receptors, thereby indirectly modulating GABA and the excitability of dopamine neurons by reducing it and other presynaptic released neurotransmitters.[1] Presynaptic inhibition is ubiquitous among sensory neurons.[2]
Function
Sensory stimuli, such as pain, proprioception, and somatosensation, are sensed by primary afferent fibers. Somatosensory neurons encode information about the body's current state (e.g. temperature, pain, pressure, position, etc.). For vertebrate animals, these primary afferent fibers form synapses onto the spinal cord, specifically in the dorsal horn area, onto a variety of downstream targets including both excitatory neurons and inhibitory neurons. Synapses between primary afferent fibers and their targets are the first opportunity for sensory information to be modulated.[3] Primary afferent fibers contain many receptors along their projections, making them amenable to complex modulation. The constant influx of environmental stimuli, as sensed by primary afferent fibers, is subject to modulation to enhance or diminish stimuli (see also: gate control theory and gain control-biological). Because there are essentially unlimited stimuli, it is imperative that these signals are appropriately filtered.
To test whether somatosensation, specifically pain, was subjected to inhibition, scientists injected a chemical into the spinal cord of a rodent to block the primary inhibitory neurotransmitter's activity (bicuculline, a GABA receptor agonist[4]). They found that pharmacologically blocking GABA receptors actually enhanced the perception of pain; in other words, GABA usually diminishes the perception of pain.[5]
The method by which GABA modulates synaptic transmission from primary afferent fibers to their downstream targets is disputed (see Mechanisms section below). Regardless of the mechanics, GABA acts in an inhibitory role to reduce the likelihood of primary afferent fiber synaptic release.
Modulating primary afferent fibers is critical to maintain general comfort. One study showed that animals without a specific type of GABA receptor on their nociceptors were hypersensitive to pain,[6] thus supporting a function of presynaptic inhibition as an analgesic. Certain pathological conditions, such as allodynia, are thought to be caused by non-modulated nociceptor firing. In addition to dampening pain, impaired presynaptic inhibition has been implicated in many neurological disorders, such as spasticity after spinal cord injury,[7] epilepsy, autism, and fragile-X syndrome.[8][9][10][11][12]
Mechanisms
Primary sensory afferents contain GABA receptors along their terminals (reviewed in:,[13] Table 1). GABA receptors are ligand-gated chloride channels, formed by the assembly of five GABA receptor subunits. In addition to the presence of GABA receptors along sensory afferent axons, the presynaptic terminal also has a distinct ionic composition that is high in chloride concentration. This is due to cation-chloride cotransporters (for example, NKCC1) that maintain highs intracellular chloride.[14]
Typically when GABA receptors are activated, it causes a chloride influx, which hyperpolarizes the cell. However, in primary afferent fibers, due to the high concentration of chloride at the presynaptic terminal and thus its altered reversal potential, GABA receptor activation actually results in a chloride efflux, and thus a resulting depolarization. This phenomenon is called primary afferent depolarization (PAD).[15][16] The GABA-induced depolarized potential at afferent axons has been demonstrated in many animals from cats to insects. Interestingly, despite the depolarized potential, GABA receptor activation along the axon still results in a reduction of neurotransmitter release and thus still is inhibitory.
There are four hypotheses which propose mechanisms behind this paradox:
- The depolarized membrane causes inactivation of voltage-gated sodium channels on the terminals and therefore the action potential is prevented from propagating.[13][17][18]
- Open GABA receptor channels act as a shunt, whereby current is dissipated of instead of being propagated to the terminals.[13][17][18][19][20][21][22][23][24]
- The depolarized membrane causes inactivation of voltage-gated calcium channels, preventing calcium influx at the synapse (which is imperative for neurotransmission).[13][18][20][21][25]
- The depolarization at the terminals generates an antidromic spike (i.e. an action potential generated in the axon and travels towards the soma), which would prevent orthodromic spikes (i.e. an action potential traveling from the cell's soma toward the axon terminals) from propagating.[19]
History of the discovery of presynaptic inhibition
1933: Grasser & Graham observed depolarization that originated in the sensory axon terminals[26]
1938: Baron & Matthews observed depolarization that originated in sensory axon terminals and the ventral root[27]
1957: Frank & Fuortes coined the term "presynaptic inhibition"[28]
1961: Eccles, Eccles, & Magni determined that the Dorsal Root Potential (DRP) originated from depolarization in sensory axon terminals [29]
References
- ↑ Oleson, Erik B. (2012-01-26). "Endocannabinoids shape accumbal encoding of cue-motivated behavior via CB1 receptor activation in the ventral tegmentum". Neuron 73 (2): 360–373. doi:10.1016/j.neuron.2011.11.018. PMID 22284189.
- ↑ "Presynaptic inhibition of olfactory sensory neurons: new mechanisms and potential functions". Chemical Senses 38 (6): 459–474. July 2013. doi:10.1093/chemse/bjt018. PMID 23761680.
- ↑ "Presynaptic Inhibition of Pain and Touch in the Spinal Cord: From Receptors to Circuits". International Journal of Molecular Sciences 22 (1): 414. January 2021. doi:10.3390/ijms22010414. PMID 33401784.
- ↑ "The Alkaloids of Fumaraceous Plants: II. Dicentra Cucullaria (L.) Bernh." (in en). Canadian Journal of Research 7 (3): 265–269. September 1932. doi:10.1139/cjr32-078. ISSN 1923-4287. Bibcode: 1932CJRes...7..265M. http://www.nrcresearchpress.com/doi/10.1139/cjr32-078.
- ↑ "Nociceptive responses to altered GABAergic activity at the spinal cord". Life Sciences 39 (18): 1667–74. November 1986. doi:10.1016/0024-3205(86)90164-5. PMID 3022091.
- ↑ "Chloride regulation in the pain pathway". Brain Research Reviews 60 (1): 149–170. April 2009. doi:10.1016/j.brainresrev.2008.12.015. PMID 19167425.
- ↑ Caron, Guillaume; Bilchak, Jadwiga N.; Côté, Marie‐Pascale (2020). "Direct evidence for decreased presynaptic inhibition evoked by PBSt group I muscle afferents after chronic SCI and recovery with step‐training in rats" (in en). The Journal of Physiology 598 (20): 4621–4642. doi:10.1113/JP280070. ISSN 0022-3751. PMID 32721039.
- ↑ "Modulation of GABAergic transmission in development and neurodevelopmental disorders: investigating physiology and pathology to gain therapeutic perspectives". Frontiers in Cellular Neuroscience 8: 119. 2014. doi:10.3389/fncel.2014.00119. PMID 24904277.
- ↑ "Fast synaptic inhibition in spinal sensory processing and pain control". Physiological Reviews 92 (1): 193–235. January 2012. doi:10.1152/physrev.00043.2010. PMID 22298656.
- ↑ "Excitation/Inhibition Imbalance in Animal Models of Autism Spectrum Disorders". Biological Psychiatry 81 (10): 838–847. May 2017. doi:10.1016/j.biopsych.2016.05.011. PMID 27450033.
- ↑ "The GABAA receptor: a novel target for treatment of fragile X?". Trends in Neurosciences 30 (8): 425–431. August 2007. doi:10.1016/j.tins.2007.06.003. PMID 17590448.
- ↑ "GABAA receptor heterogeneity, function, and implications for epilepsy". Neurology 68 (8): 612–614. February 2007. doi:10.1212/01.wnl.0000255669.83468.dd. PMID 17310035.
- ↑ 13.0 13.1 13.2 13.3 "Spinal presynaptic inhibition in pain control". Neuroscience 283: 95–106. December 2014. doi:10.1016/j.neuroscience.2014.09.032. PMID 25255936.
- ↑ "Roles of the cation-chloride cotransporters in neurological disease". Nature Clinical Practice. Neurology 4 (9): 490–503. September 2008. doi:10.1038/ncpneuro0883. PMID 18769373.
- ↑ "Chloride regulation in the pain pathway". Brain Research Reviews 60 (1): 149–170. April 2009. doi:10.1016/j.brainresrev.2008.12.015. PMID 19167425.
- ↑ "Dorsal root potentials and dorsal root reflexes: a double-edged sword". Experimental Brain Research 124 (4): 395–421. February 1999. doi:10.1007/s002210050637. PMID 10090653.
- ↑ 17.0 17.1 "Shunting versus inactivation: analysis of presynaptic inhibitory mechanisms in primary afferents of the crayfish". The Journal of Neuroscience 19 (14): 6079–6089. July 1999. doi:10.1523/JNEUROSCI.19-14-06079.1999. PMID 10407044.
- ↑ 18.0 18.1 18.2 "John Eccles' studies of spinal cord presynaptic inhibition". Progress in Neurobiology 78 (3–5): 189–214. 2006-02-01. doi:10.1016/j.pneurobio.2006.02.007. PMID 16650518.
- ↑ 19.0 19.1 "Presynaptic inhibition and antidromic spikes in primary afferents of the crayfish: a computational and experimental analysis". The Journal of Neuroscience 21 (3): 1007–1021. February 2001. doi:10.1523/JNEUROSCI.21-03-01007.2001. PMID 11157086.
- ↑ 20.0 20.1 "Peripheral GABAergic inhibition of spider mechanosensory afferents". The European Journal of Neuroscience 16 (1): 96–104. July 2002. doi:10.1046/j.1460-9568.2002.02065.x. PMID 12153534.
- ↑ 21.0 21.1 "Shunting versus inactivation: simulation of GABAergic inhibition in spider mechanoreceptors suggests that either is sufficient". Neuroscience Research 55 (2): 189–196. June 2006. doi:10.1016/j.neures.2006.03.002. PMID 16616790.
- ↑ "Presynaptic receptors". Annual Review of Pharmacology and Toxicology 38: 201–227. 1998. doi:10.1146/annurev.pharmtox.38.1.201. PMID 9597154.
- ↑ "Properties of the GABAA receptor of rat posterior pituitary nerve terminals". Journal of Neurophysiology 73 (3): 1135–1144. March 1995. doi:10.1152/jn.1995.73.3.1135. PMID 7608760.
- ↑ "GABAA receptor activation and the excitability of nerve terminals in the rat posterior pituitary". The Journal of Physiology 483 (3): 583–595. March 1995. doi:10.1113/jphysiol.1995.sp020608. PMID 7776245.
- ↑ "A simulation of action potentials in synaptic boutons during presynaptic inhibition". Journal of Neurophysiology 71 (2): 538–549. February 1994. doi:10.1152/jn.1994.71.2.538. PMID 8176423.
- ↑ "Potentials produced in the spinal cord by stimulation of dorsal roots". American Journal of Physiology 103 (2): 303–320. January 1933. doi:10.1152/ajplegacy.1933.103.2.303.
- ↑ "The interpretation of potential changes in the spinal cord". The Journal of Physiology 92 (3): 276–321. April 1938. doi:10.1113/jphysiol.1938.sp003603. PMID 16994975.
- ↑ "Presynaptic and Postsynaptic inhibition of monsynaptic reflexes". Federation Proceedings 16: 39–40. 1957.
- ↑ "Central inhibitory action attributable to presynaptic depolarization produced by muscle afferent volleys". The Journal of Physiology 159 (1): 147–166. November 1961. doi:10.1113/jphysiol.1961.sp006798. PMID 13889050.
 |