Chemistry:Anthraquinone process
The anthraquinone process is a process for the production of hydrogen peroxide, which was developed by BASF. The industrial production of hydrogen peroxide is based on the reduction of oxygen, as in the direct synthesis from the elements. Instead of hydrogen itself, however, a 2-alkyl-anthrahydroquinone, which is generated before from the corresponding 2-alkyl-anthraquinone by catalytic hydrogenation with palladium is used. Oxygen and the organic phase react under formation of the anthraquinone and hydrogen peroxide. Among other alkyl groups (R) ethyl- and tert-butyl- are used, e.g., 2-ethylanthraquinone.[1][2]
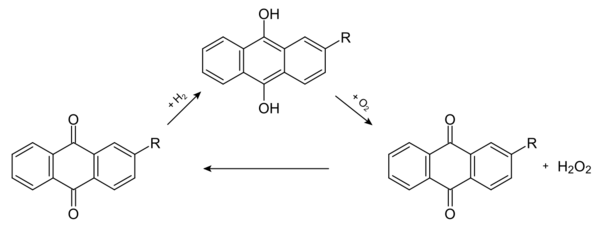
The hydrogen peroxide is then extracted with water and in a second step separated by fractional distillation from the water. The hydrogen peroxide accumulates as sump product. The anthraquinone acts as a catalyst, the overall reaction equation is therefore:
- H2 + O2 → H2O2
If ozone is used instead of oxygen, dihydrogen trioxide can be produced by this method.[3]
References
- ↑ Goor, G.; Glenneberg, J.; Jacobi, S. (2007). "Hydrogen Peroxide". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a13_443.pub2. ISBN 978-3527306732.
- ↑ Römpp CD 2006, Georg Thieme Verlag 2006
- ↑ Plesničar, Božo (2005). "Progress in the Chemistry of Dihydrogen Trioxide (HOOOH)". Acta Chimica Slovenica 52: 1–12. http://acta-arhiv.chem-soc.si/52/52-1-1.pdf.
External links
 |
