Chemistry:Atom transfer radical polymerization
Atom transfer radical polymerization (ATRP) is an example of a reversible-deactivation radical polymerization. Like its counterpart, ATRA, or atom transfer radical addition, ATRP is a means of forming a carbon-carbon bond with a transition metal catalyst. Polymerization from this method is called atom transfer radical addition polymerization (ATRAP). As the name implies, the atom transfer step is crucial in the reaction responsible for uniform polymer chain growth. ATRP (or transition metal-mediated living radical polymerization) was independently discovered by Mitsuo Sawamoto[1] and by Krzysztof Matyjaszewski and Jin-Shan Wang in 1995.[2][3]
- The following scheme presents a typical ATRP reaction:
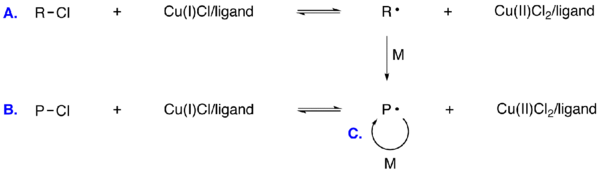
of the radicals involves reversible atom transfer or reversible group transfer catalyzed usually,
though not exclusively, by transition-metal complexes.[4]
Overview of ATRP
ATRP usually employs a transition metal complex as the catalyst with an alkyl halide as the initiator (R-X). Various transition metal complexes, namely those of Cu, Fe, Ru, Ni, and Os, have been employed as catalysts for ATRP. In an ATRP process, the dormant species is activated by the transition metal complex to generate radicals via one electron transfer process. Simultaneously the transition metal is oxidized to higher oxidation state. This reversible process rapidly establishes an equilibrium that is predominately shifted to the side with very low radical concentrations. The number of polymer chains is determined by the number of initiators. Each growing chain has the same probability to propagate with monomers to form living/dormant polymer chains (R-Pn-X). As a result, polymers with similar molecular weights and narrow molecular weight distribution can be prepared.
ATRP reactions are very robust in that they are tolerant of many functional groups like allyl, amino, epoxy, hydroxy, and vinyl groups present in either the monomer or the initiator.[5] ATRP methods are also advantageous due to the ease of preparation, commercially available and inexpensive catalysts (copper complexes), pyridine-based ligands, and initiators (alkyl halides).[6]

Components of normal ATRP
There are five important variable components of atom transfer radical polymerizations. They are the monomer, initiator, catalyst, ligand, and solvent. The following section breaks down the contributions of each component to the overall polymerization.
Monomer
Monomers typically used in ATRP are molecules with substituents that can stabilize the propagating radicals; for example, styrenes, (meth)acrylates, (meth)acrylamides, and acrylonitrile.[7] ATRP is successful at leading to polymers of high number average molecular weight and low dispersity when the concentration of the propagating radical balances the rate of radical termination. Yet, the propagating rate is unique to each individual monomer. Therefore, it is important that the other components of the polymerization (initiator, catalyst, ligand, and solvent) are optimized in order for the concentration of the dormant species to be greater than that of the propagating radical while being low enough as to prevent slowing down or halting the reaction.[8][9]
Initiator
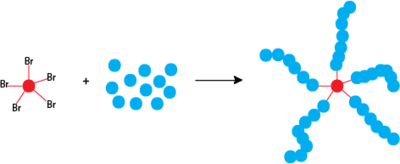
The number of growing polymer chains is determined by the initiator. To ensure a low polydispersity and a controlled polymerization, the rate of initiation must be as fast or preferably faster than the rate of propagation [10] Ideally, all chains will be initiated in a very short period of time and will be propagated at the same rate. Initiators are typically chosen to be alkyl halides whose frameworks are similar to that of the propagating radical.[8] Alkyl halides such as alkyl bromides are more reactive than alkyl chlorides. Both offer good molecular weight control.[8][9] The shape or structure of the initiator influences polymer architecture. For example, initiators with multiple alkyl halide groups on a single core can lead to a star-like polymer shape.[11] Furthermore, α-functionalized ATRP initiators can be used to synthesize hetero-telechelic polymers with a variety of chain-end groups[12]
Catalyst
The catalyst is the most important component of ATRP because it determines the equilibrium constant between the active and dormant species. This equilibrium determines the polymerization rate. An equilibrium constant that is too small may inhibit or slow the polymerization while an equilibrium constant that is too large leads to a wide distribution of chain lengths.[9]
There are several requirements for the metal catalyst:
- There needs to be two accessible oxidation states that are differentiated by one electron
- The metal center needs to have reasonable affinity for halogens
- The coordination sphere of the metal needs to be expandable when it is oxidized as to accommodate the halogen
- The transition metal catalyst should not lead to significant side reactions, such as irreversible coupling with the propagating radicals and catalytic radical termination
The most studied catalysts are those that include copper, which has shown the most versatility with successful polymerizations for a wide selection of monomers.
Ligand
One of the most important aspects in an ATRP reaction is the choice of ligand which is used in combination with the traditionally copper halide catalyst to form the catalyst complex. The main function of the ligand is to solubilize the copper halide in whichever solvent is chosen and to adjust the redox potential of the copper.[13] This changes the activity and dynamics of the halogen exchange reaction and subsequent activation and deactivation of the polymer chains during polymerization, therefore greatly affecting the kinetics of the reaction and the degree of control over the polymerization. Different ligands should be chosen based on the activity of the monomer and the choice of metal for the catalyst. As copper halides are primarily used as the catalyst, amine based ligands are most commonly chosen. Ligands with higher activities are being investigated as ways to potentially decrease the concentration of catalyst in the reaction since a more active catalyst complex would lead to a higher concentration of deactivator in the reaction. However, a too active catalyst can lead to a loss of control and increase the polydispersity of the resulting polymer.
Solvents
Toluene, 1,4-dioxane, xylene, anisole, DMF, DMSO, water, methanol, acetonitrile, or even the monomer itself (described as a bulk polymerization) are commonly used.
Kinetics of normal ATRP
- Reactions in atom transfer radical polymerization
- Initiation
- Quasi-steady state
- Other chain breaking reactions () should also be considered.
ATRP equilibrium constant
The radical concentration in normal ATRP can be calculated via the following equation:
It is important to know the KATRP value to adjust the radical concentration. The KATRP value depends on the homo-cleavage energy of the alkyl halide and the redox potential of the Cu catalyst with different ligands. Given two alkyl halides (R1-X and R2-X) and two ligands (L1 and L2), there will be four combinations between different alkyl halides and ligands. Let KijATRP refer to the KATRP value for Ri-X and Lj. If we know three of these four combinations, the fourth one can be calculated as:
The KATRP values for different alkyl halides and different Cu catalysts can be found in literature.[14]
Solvents have significant effects on the KATRP values. The KATRP value increases dramatically with the polarity of the solvent for the same alkyl halide and the same Cu catalyst.[15] The polymerization must take place in solvent/monomer mixture, which changes to solvent/monomer/polymer mixture gradually. The KATRP values could change 10000 times by switching the reaction medium from pure methyl acrylate to pure dimethyl sulfoxide.[16]
Activation and deactivation rate coefficients
Deactivation rate coefficient, kd, values must be sufficiently large to obtain low dispersity. The direct measurement of kd is difficult though not impossible. In most cases, kd may be calculated from known KATRP and ka.[14][17][18] Cu complexes providing very low kd values are not recommended for use in ATRP reactions.
Retention of chain end functionality
High level retention of chain end functionality is typically desired. However, the determination of the loss of chain end functionality based on 1H NMR and mass spectroscopy methods cannot provide precise values. As a result, it is difficult to identify the contributions of different chain breaking reactions in ATRP. One simple rule in ATRP comprises the principle of halogen conservation.[19] Halogen conservation means the total amount of halogen in the reaction systems must remain as a constant. From this rule, the level of retention of chain end functionality can be precisely determined in many cases. The precise determination of the loss of chain end functionality enabled further investigation of the chain breaking reactions in ATRP.[20]
Advantages and disadvantages of ATRP
Advantages
ATRP enables the polymerization of a wide variety of monomers with different chemical functionalities, proving to be more tolerant of these functionalities than ionic polymerizations. It provides increased control of molecular weight, molecular architecture and polymer composition while maintaining a low polydispersity (1.05-1.2). The halogen remaining at the end of the polymer chain after polymerization allows for facile post-polymerization chain-end modification into different reactive functional groups. The use of multi-functional initiators facilitates the synthesis of lower-arm star polymers and telechelic polymers. External visible light stimulation ATRP has a high responding speed and excellent functional group tolerance.[21]
Disadvantages
The most significant drawback of ATRP is the high concentrations of catalyst required for the reaction. This catalyst standardly consists of a copper halide and an amine-based ligand. The removal of the copper from the polymer after polymerization is often tedious and expensive, limiting ATRP's use in the commercial sector.[22] However, researchers are currently developing methods which would limit the necessity of the catalyst concentration to ppm. ATRP is also a traditionally air-sensitive reaction normally requiring freeze-pump thaw cycles. However, techniques such as Activator Generated by Electron Transfer (AGET) ATRP provide potential alternatives which are not air-sensitive.[23] A final disadvantage is the difficulty of conducting ATRP in aqueous media.
Different ATRP methods
Activator regeneration ATRP methods
In a normal ATRP, the concentration of radicals is determined by the KATRP value, concentration of dormant species, and the [CuI]/[CuII] ratio. In principle, the total amount of Cu catalyst should not influence polymerization kinetics. However, the loss of chain end functionality slowly but irreversibly converts CuI to CuII. Thus initial [CuI]/[I] ratios are typically 0.1 to 1. When very low concentrations of catalysts are used, usually at the ppm level, activator regeneration processes are generally required to compensate the loss of CEF and regenerate a sufficient amount of CuI to continue the polymerization. Several activator regeneration ATRP methods were developed, namely ICAR ATRP, ARGET ATRP, SARA ATRP, eATRP, and photoinduced ATRP. The activator regeneration process is introduced to compensate the loss of chain end functionality, thus the cumulative amount of activator regeneration should roughly equal the total amount of the loss of chain end functionality.

ICAR ATRP
Initiators for continuous activator regeneration (ICAR) is a technique that uses conventional radical initiators to continuously regenerate the activator, lowering its required concentration from thousands of ppm to <100 ppm; making it an industrially relevant technique.
ARGET ATRP
Activators regenerated by electron transfer (ARGET) employs non-radical forming reducing agents for regeneration of CuI. A good reducing agent (e.g. hydrazine, phenols, sugars, ascorbic acid) should only react with CuII and not with radicals or other reagents in the reaction mixture.
SARA ATRP
A typical SARA ATRP employs Cu0 as both supplemental activator and reducing agent (SARA). Cu0 can activate alkyl halide directly but slowly. Cu0 can also reduce CuII to CuI. Both processes help to regenerate CuI activator. Other zerovalent metals, such as Mg, Zn, and Fe, have also been employed for Cu-based SARA ATRP.
eATRP
In eATRP the activator CuI is regenerated via electrochemical process. The development of eATRP enables precise control of the reduction process and external regulation of the polymerization. In an eATRP process, the redox reaction involves two electrodes. The CuII species is reduced to CuI at the cathode. The anode compartment is typically separated from the polymerization environment by a glass frit and a conductive gel. Alternatively, a sacrificial aluminum counter electrode can be used, which is directly immersed in the reaction mixture.
Photoinduced ATRP
The direct photo reduction of transition metal catalysts in ATRP and/or photo assistant activation of alkyl halide is particularly interesting because such a procedure will allow performing of ATRP with ppm level of catalysts without any other additives.
Other ATRP methods
Reverse ATRP
In reverse ATRP, the catalyst is added in its higher oxidation state. Chains are activated by conventional radical initiators (e.g. AIBN) and deactivated by the transition metal. The source of transferable halogen is the copper salt, so this must be present in concentrations comparable to the transition metal.
SR&NI ATRP
A mixture of radical initiator and active (lower oxidation state) catalyst allows for the creation of block copolymers (contaminated with homopolymer) which is impossible using standard reverse ATRP. This is called SR&NI (simultaneous reverse and normal initiation ATRP).
AGET ATRP
Activators generated by electron transfer uses a reducing agent unable to initiate new chains (instead of organic radicals) as regenerator for the low-valent metal. Examples are metallic copper, tin(II), ascorbic acid, or triethylamine. It allows for lower concentrations of transition metals, and may also be possible in aqueous or dispersed media.
Hybrid and bimetallic systems
This technique uses a variety of different metals/oxidation states, possibly on solid supports, to act as activators/deactivators, possibly with reduced toxicity or sensitivity.[24][25] Iron salts can, for example, efficiently activate alkyl halides but requires an efficient Cu(II) deactivator which can be present in much lower concentrations (3–5 mol%)
Metal-free ATRP
Trace metal catalyst remaining in the final product has limited the application of ATRP in biomedical and electronic fields. In 2014, Craig Hawker and coworkers developed a new catalysis system involving photoredox reaction of 10-phenothiazine. The metal-free ATRP has been demonstrated to be capable of controlled polymerization of methacrylates.[26] This technique was later expanded to polymerization of acrylonitrile by Matyjaszewski et al.[27]
Mechano/sono-ATRP
Mechano/sono-ATRP uses mechanical forces, typically ultrasonic agitation, as an external stimulus to induce the (re)generation of activators in ATRP. Esser-Kahn, et al. demonstrated the first example of mechanoATRP using the piezoelectricity of barium titanate to reduce Cu(II) species.[28] Matyjaszewski, et al. later improved the technique by using nanometer-sized and/or surface-functionalized barium titanate or zinc oxide particles, achieving superior rate and control of polymerization, as well as temporal control, with ppm-level of copper catalysts.[29][30] In addition to peizoelectric particles, water and carbonates were found to mediate mechano/sono-ATRP. Mechochemically homolyzed water molecules undergoes radical addition to monomers, which in turn reduces Cu(II) species.[31] Mechanically unstable Cu(II)-carbonate complexes formed in the presence to insoluble carbonates, which oxidizes dimethylsulfoxide, the solvent molecules, to generate Cu(I) species and carbon dioxide.[32]
Biocatalytic ATRP
Metalloenzymes have been used for the first time as ATRP catalysts, in parallel and independently, by the research teams of Fabio Di Lena[33] and Nico Bruns.[34] This pioneering work has paved the way to the emerging field of biocatalytic reversible-deactivation radical polymerization.[35][36]
Polymers synthesized through ATRP
- Polystyrene
- Poly (methyl methacrylate)
- Polyacrylamide
See also
- Heteropolymer
- Radical
- Reversible addition−fragmentation chain-transfer polymerization
- Nitroxide mediated radical polymerization
External links
References
- ↑ Kato, M; Kamigaito, M; Sawamoto, M; Higashimura, T (1995). "Polymerization of Methyl Methacrylate with the Carbon Tetrachloride / Dichlorotris-(triphenylphosphine)ruthenium(II) / Methylaluminum Bis(2,6-di-tert-butylphenoxide) Initiating System: Possibility of Living Radical Polymerization". Macromolecules 28 (5): 1721–1723. doi:10.1021/ma00109a056. Bibcode: 1995MaMol..28.1721K.
- ↑ Wang, J-S; Matyjaszewski, K (1995). "Controlled/"living" radical polymerization. Atom transfer radical polymerization in the presence of transition-metal complexes". J. Am. Chem. Soc. 117 (20): 5614–5615. doi:10.1021/ja00125a035.
- ↑ "The 2011 Wolf Prize in Chemistry". Wolf Fund. http://www.wolffund.org.il/cat.asp?id=15&cat_title=CHEMISTRY. Retrieved 21 February 2011.
- ↑ Jenkins, Aubrey D.; Jones, Richard G.; Moad, Graeme (2010). "Terminology for reversible-deactivation radical polymerization previously called "controlled" radical or "living" radical polymerization (IUPAC Recommendations 2010)". Pure and Applied Chemistry 82 (2): 483–491. doi:10.1351/PAC-REP-08-04-03. http://pac.iupac.org/publications/pac/pdf/2010/pdf/8202x0483.pdf.
- ↑ Cowie, J. M. G.; Arrighi, V. In Polymers: Chemistry and Physics of Modern Materials; CRC Press Taylor and Francis Group: Boca Raton, Fl, 2008; 3rd Ed., pp. 82–84 ISBN 0849398134
- ↑ Matyjaszewski, K.. "Fundamentals of ATRP Research". http://www.chem.cmu.edu/groups/maty/about/research/03.html.
- ↑ Patten, T. E; Matyjaszewski, K (1998). "Atom Transfer Radical Polymerization and the Synthesis of Polymeric Materials". Adv. Mater. 10 (12): 901–915. doi:10.1002/(sici)1521-4095(199808)10:12<901::aid-adma901>3.0.co;2-b.
- ↑ 8.0 8.1 8.2 Odian, G. In Radical Chain Polymerization; Principles of Polymerization; Wiley-Interscience: Staten Island, New York, 2004; Vol. , pp 316–321.
- ↑ 9.0 9.1 9.2 Matyjaszewski, Krzysztof; Xia, Jianhui (2001). "Atom Transfer Radical Polymerization". Chem. Rev. 101 (9): 2921–90. doi:10.1021/cr940534g. ISSN 0009-2665. PMID 11749397.
- ↑ <!- -Not stated- ->. "Initiators". Carnegie Mellon University. http://www.cmu.edu/maty/atrp-how/initiators.html.
- ↑ Jakubowski, Wojciech. "Complete Tools for the Synthesis of Well-Defined Functionalized Polymers via ATRP". Sigma-Aldrich. http://www.sigmaaldrich.com/materials-science/polymer-science/atrp.html. Retrieved 21 July 2010.
- ↑ <!- -Not stated- ->. "Use of Functional ATRP Initiators". Carnegie Mellon University. http://www.cmu.edu/maty/materials/Incorporation-of-functional-groups-into-polymers/use-of-functional-atrp-initiators.html.
- ↑ <!- -Not stated- ->. "Structural Characterization of an ATRP Catalyst Complex". Carnegie Mellon University. https://www.cmu.edu/maty/chem/catalyst-development/structural-characterization.html.
- ↑ 14.0 14.1 Tang, W; Kwak, Y; Braunecker, W; Tsarevsky, N V; Coote, M L; Matyjaszewski, K (2008). "Understanding Atom Transfer Radical Polymerization: Effect of Ligand and Initiator Structures on the Equilibrium Constants". J. Am. Chem. Soc. 130 (32): 10702–10713. doi:10.1021/ja802290a. PMID 18642811.
- ↑ Braunecker, W; Tsarevsky, N V; Gennaro, A; Matyjaszewski, K (2009). "Thermodynamic Components of the Atom Transfer Radical Polymerization Equilibrium: Quantifying Solvent Effects". Macromolecules 42 (17): 6348–6360. doi:10.1021/ma901094s. Bibcode: 2009MaMol..42.6348B.
- ↑ Wang, Y; Kwak, Y; Buback, J; Buback, M; Matyjaszewski, K (2012). "Determination of ATRP Equilibrium Constants under Polymerization Conditions". ACS Macro Lett. 1 (12): 1367–1370. doi:10.1021/mz3005378. https://figshare.com/articles/Determination_of_ATRP_Equilibrium_Constants_under_Polymerization_Conditions/2459842.
- ↑ Tang, W; Matyjaszewski, K (2007). "Effects of Initiator Structure on Activation Rate Constants in ATRP". Macromolecules 40 (6): 1858–1863. doi:10.1021/ma062897b. Bibcode: 2007MaMol..40.1858T.
- ↑ Tang, W; Matyjaszewski, K (2006). "Effect of Ligand Structure on Activation Rate Constants in ATRP". Macromolecules 39 (15): 4953–4959. doi:10.1021/ma0609634. Bibcode: 2006MaMol..39.4953T.
- ↑ Wang, Y; Zhong, M; Zhang, Y; Magenau, A J D; Matyjaszewski, K (2012). "Halogen Conservation in Atom Transfer Radical Polymerization". Macromolecules 45 (21): 8929–8932. doi:10.1021/ma3018958. Bibcode: 2012MaMol..45.8929W.
- ↑ Wang, Y; Soerensen, N; Zhong, M; Schroeder, H; Buback, M; Matyjaszewski, K (2013). "Improving the "Livingness" of ATRP by Reducing Cu Catalyst Concentration". Macromolecules 46 (3): 689–691. doi:10.1021/ma3024393. Bibcode: 2013MaMol..46..683W.
- ↑ "Atom-Transfer Radical-Polymerization (ATRP) – Artificial Intelligence for Chemistry" (in en-US). http://ai4c.com/2019/11/19/atom-transfer-radical-polymerization-atrp/.
- ↑ Borman, Stu (October 30, 2006). "Polymers with Safe Amounts of Copper". Chemical & Engineering News 84 (43): 40–41. doi:10.1021/cen-v084n044.p040. https://pubs.acs.org/cen/science/84/8444sci1.html. Retrieved November 30, 2018.
- ↑ Siegwart, Daniel; Kwan Oh, Jung; Matyjaszewski, Krzysztof (January 1, 2012). "ATRP in the design of functional materials for biomedical applications". Progress in Polymer Science 37 (1): 18–37. doi:10.1016/j.progpolymsci.2011.08.001. PMID 23525884.
- ↑ Xiong, De'an; He, Zhenping (15 January 2010). "Modulating the catalytic activity of Au/micelles by tunable hydrophilic channels". Journal of Colloid and Interface Science 341 (2): 273–279. doi:10.1016/j.jcis.2009.09.045. PMID 19854448. Bibcode: 2010JCIS..341..273X.
- ↑ Chen, Xi et al. (5 August 2008). "Core-shell-corona Au-micelle composites with a tunable smart hybrid shell". Langmuir 24 (15): 8198–8204. doi:10.1021/la800244g. PMID 18576675.
- ↑ Treat, Nicolas; Sprafke, Hazel; Kramer, John; Clark, Paul; Barton, Bryan; Read de Alaniz, Javier; Fors, Brett; Hawker, Craig (2014). "Metal-Free Atom Transfer Radical Polymerization". Journal of the American Chemical Society 136 (45): 16096–16101. doi:10.1021/ja510389m. PMID 25360628. http://www.escholarship.org/uc/item/5vm8s0vp.
- ↑ Pan, Xiangcheng; Lamson, Melissa; Yan, Jiajun; Matyjaszewski, Krzysztof (17 February 2015). "Photoinduced Metal-Free Atom Transfer Radical Polymerization of Acrylonitrile". ACS Macro Letters 4 (2): 192–196. doi:10.1021/mz500834g.
- ↑ Mohapatra, Hemakesh; Kleiman, Maya; Esser-Kahn, Aaron Palmer (24 October 2016). "Mechanically controlled radical polymerization initiated by ultrasound". Nature Chemistry 9 (2): 135–139. doi:10.1038/nchem.2633.
- ↑ Wang, Zhenhua; Pan, Xiangcheng; Yan, Jiajun; Dadashi-Silab, Sajjad; Xie, Guojun; Zhang, Jianan; Wang, Zhanhua; Xia, Hesheng et al. (28 April 2017). "Temporal Control in Mechanically Controlled Atom Transfer Radical Polymerization Using Low ppm of Cu Catalyst". ACS Macro Letters 6 (5): 546–549. doi:10.1021/acsmacrolett.7b00152.
- ↑ Wang, Zhenhua; Pan, Xiangcheng; Li, Lingchun; Fantin, Marco; Yan, Jiajun; Wang, Zongyu; Wang, Zhanhua; Xia, Hesheng et al. (4 October 2017). "Enhancing Mechanically Induced ATRP by Promoting Interfacial Electron Transfer from Piezoelectric Nanoparticles to Cu Catalysts". Macromolecules 50 (20): 7940–7948. doi:10.1021/acs.macromol.7b01597. Bibcode: 2017MaMol..50.7940W.
- ↑ Wang, Zhenhua; Wang, Zhanhua; Pan, Xiangcheng; Fu, Liye; Lathwal, Sushil; Olszewski, Mateusz; Yan, Jiajun; Enciso, Alan E. et al. (20 March 2018). "Ultrasonication-Induced Aqueous Atom Transfer Radical Polymerization" (in en). ACS Macro Letters 7 (3): 275–280. doi:10.1021/acsmacrolett.8b00027. ISSN 2161-1653.
- ↑ Wang, Zhenhua; Lorandi, Francesca; Fantin, Marco; Wang, Zongyu; Yan, Jiajun; Wang, Zhanhua; Xia, Hesheng; Matyjaszewski, Krzysztof (22 January 2019). "Atom Transfer Radical Polymerization Enabled by Sonochemically Labile Cu-carbonate Species". ACS Macro Letters 8 (2): 161–165. doi:10.1021/acsmacrolett.9b00029. https://figshare.com/articles/Atom_Transfer_Radical_Polymerization_Enabled_by_Sonochemically_Labile_Cu-carbonate_Species/7615799.
- ↑ Ng, Yeap-Hung; Lena, Fabio di; Chai, Christina L. L. (2011-05-24). "PolyPEGA with predetermined molecular weights from enzyme-mediated radical polymerization in water" (in en). Chemical Communications 47 (22): 6464–6466. doi:10.1039/C1CC10989H. ISSN 1364-548X. https://pubs.rsc.org/en/content/articlelanding/2011/cc/c1cc10989h.
- ↑ Sigg, Severin J.; Seidi, Farzad; Renggli, Kasper; Silva, Tilana B.; Kali, Gergely; Bruns, Nico (2011-11-01). "Horseradish Peroxidase as a Catalyst for Atom Transfer Radical Polymerization: Horseradish Peroxidase as a Catalyst for Atom Transfer …" (in en). Macromolecular Rapid Communications 32 (21): 1710–1715. doi:10.1002/marc.201100349. https://onlinelibrary.wiley.com/doi/10.1002/marc.201100349.
- ↑ Enzyme Catalysis for Reversible Deactivation Radical Polymerization. Ruoyu Li, Weina Kong, Zesheng An. Angew. Chem. Int. Ed. 2022, 61, e202202033 https://doi.org/10.1002/ange.202202033
- ↑ Li, Ruoyu; Kong, Weina; An, Zesheng (2023-02-14). "Controlling Radical Polymerization with Biocatalysts" (in en). Macromolecules 56 (3): 751–761. doi:10.1021/acs.macromol.2c02307. ISSN 0024-9297. https://pubs.acs.org/doi/10.1021/acs.macromol.2c02307.
 |
