Chemistry:BOLD-100
 BOLD-100 Chemical Structure | |
| Clinical data | |
|---|---|
| Routes of administration | Intravenous |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChEBI | |
| Chemical and physical data | |
| 3D model (JSmol) | |
| |
| |
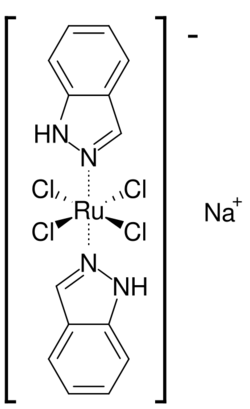
BOLD-100, or sodium trans-[tetrachlorobis (1H-indazole)ruthenate(III)], is a ruthenium-based anti-cancer therapeutic in clinical development. As of November 2021, BOLD-100 was being tested in a Phase 1b clinical trial in patients with advanced gastrointestinal cancers in combination with the chemotherapy regimen FOLFOX.[1] BOLD-100 is being developed by Bold Therapeutics Inc.[2]
Structure
BOLD-100 has an octahedral structure with two trans indazoles and four chloride ligands in the equatorial plane. The primary cation for BOLD-100 is sodium. BOLD-100’s impurity profile contains trace quantities of cesium [3]
BOLD-100 derivatives
BOLD-100 is sodium trans-[tetrachlorobis (1H-indazole) ruthenate(III)] with cesium as an intermediate salt form.[4] BOLD-100 was developed from the closely related ruthenium molecule KP1339 (also known as IT-139 or NKP-1339) which is also sodium trans-[tetrachlorobis (1H-indazole) ruthenate(III)], but has different manufacturing methods and purity profiles. The names are often used interchangeably.[5]
The precursor molecule to BOLD-100 is KP1019, which is the indazole salt equivalent. KP1019 previously entered Phase 1 clinical trials but development was halted due to low solubility in water, leading to the development of KP1339 and BOLD-100 which are readily soluble in water. KP1019 and KP1339 were invented by Dr. Keppler at the University of Vienna.[6]
Synthesis
Synthesis of BOLD-100 is accomplished by treating RuCl3 with an excess of 1H-indazole in a concentrated aqueous HCl solution. The resulting indazolium salt is treated with CsCl, and a salt exchange is performed that converts the cesium salt to the final sodium salt. The drug product is prepared as a lyophilized powder for parenteral administration.[citation needed]
Mechanism of action
BOLD-100 kills cancer cells through multiple mechanisms, leading to cell death through apoptosis. BOLD-100 inhibits GRP78 and alters the unfolded protein response (UPR), while also inducing reactive oxygen species (ROS), leading to DNA damage.[7] BOLD-100 can synergize with cytotoxic chemotherapies and targeted agents to improve cancer cell death.[7] BOLD-100 also causes immunogenic cell death in colon cancer organoids.[8]
Clinical development
The precursor molecule to BOLD-100, KP1339 was tested in a Phase 1 monotherapy clinical trial in heavily pretreated patients with advanced cancers. In this dose escalation study, KP1339 was administered to 46 patients with doses ranging from 20 mg/m2 to 780 mg/m2. KP1339 was well tolerated, with the treatment-emergent adverse events occurring in >20% of patients being nausea, fatigue, vomiting, anaemia and dehydration. These adverse events were mainly grade 2 or lower. In the 38 efficacy-evaluable patients, nine patients achieved stable disease and 1 patient had a durable partial response. 625 mg/m2 was determined to be the recommended Phase 2 dose.[9] BOLD-100 is being tested in a Phase 1b clinical trial in combination with the chemotherapy regimen FOLFOX (5-fluorouracil, leucovorin, and oxaliplatin) for the treatment of gastrointestinal cancers, including gastric, pancreatic, colon and bile duct cancer. This trial includes a dose escalation phase followed by a cohort expansion and is expected to enroll 80 patients.[1]
References
- ↑ 1.0 1.1 Clinical trial number NCT04421820 NCT04421820 for "BOLD-100 in combination with FOLFOX for the treatment of advanced solid tumours." at ClinicalTrials.gov
- ↑ "Technology". Bold Therapeutics. 2021. https://www.bold-therapeutics.com/technology.
- ↑ Vojkovsky T, Sill K, Carie A, "Manufacture of trans-[tetrachlorobis(1H-inadazole)ruthenate(III)] and compositions thereof", US patent 10611787, published 15 November 2018, assigned to Bold Therapeutics, Inc.
- ↑ "he Anticancer Ruthenium Compound BOLD-100 Targets Glycolysis and Generates a Metabolic Vulnerability towards Glucose Deprivation. Pharmaceutics". Pharmaceutics 14 (2): 238. January 20, 2022. doi:10.3390/pharmaceutics14020238. PMID 35213972.
- ↑ "Inhibition of DNA Repair Pathways and Induction of ROS Are Potential Mechanisms of Action of the Small Molecule Inhibitor BOLD-100 in Breast Cancer". Cancers 12 (9): 2647. September 2020. doi:10.3390/cancers12092647. PMID 32947941.
- ↑ "KP1019, a new redox-active anticancer agent--preclinical development and results of a clinical phase I study in tumor patients". Chemistry & Biodiversity 5 (10): 2140–2155. October 2008. doi:10.1002/cbdv.200890195. PMID 18972504.
- ↑ 7.0 7.1 "Suppression of stress induction of the 78-kilodalton glucose regulated protein (GRP78) in cancer by IT-139, an anti-tumor ruthenium small molecule inhibitor". Oncotarget 9 (51): 29698–29714. July 2018. doi:10.18632/oncotarget.25679. PMID 30038714.
- ↑ "First-in-class ruthenium anticancer drug (KP1339/IT-139) induces an immunogenic cell death signature in colorectal spheroids in vitro". Metallomics 11 (6): 1044–1048. June 2019. doi:10.1039/c9mt00051h. PMID 30942231.
- ↑ "Safety and activity of IT-139, a ruthenium-based compound, in patients with advanced solid tumours: a first-in-human, open-label, dose-escalation phase I study with expansion cohort". ESMO Open 1 (6): e000154. 2016. doi:10.1136/esmoopen-2016-000154. PMID 28848672.
 |

