Chemistry:Barbier–Wieland degradation
From HandWiki
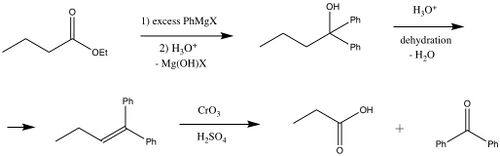
The Barbier–Wieland degradation is a procedure for shortening the carbon chain of a carboxylic acid by one carbon. It only works when the carbon adjacent to the carboxyl is a simple methylene bridge (an aliphatic carbon with no substituents). The reaction sequence involves conversion of the carboxyl and alpha carbon into an alkene, which is then cleaved by oxidation to convert the former alpha position into a carboxyl itself.[1][2]
References
- ↑ Byron Riegel, Byron; Moffett, R. B.; McIntosh A. V. (1955). "nor-Desoxycholic Acid". Organic Syntheses 24: 38. http://www.orgsyn.org/demo.aspx?prep=CV3P0234.; Collective Volume, 3, pp. 234
- ↑ Byron Riegel, Byron; Moffett, R. B.; McIntosh A. V. (1955). "3,12-Diacetoxy-bisnor-cholanyldiphenylethylene". Organic Syntheses 24: 41. http://www.orgsyn.org/demo.aspx?prep=CV3P0237.; Collective Volume, 3, pp. 237
 |

