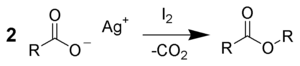Chemistry:Hunsdiecker reaction
| Hunsdiecker reaction | |
|---|---|
| Named after | Heinz Hunsdiecker Cläre Hunsdiecker Alexander Borodin |
| Reaction type | Substitution reaction |
| Identifiers | |
| Organic Chemistry Portal | hunsdiecker-reaction |
| RSC ontology ID | RXNO:0000106 |
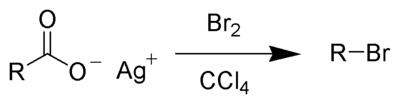
The Hunsdiecker reaction (also called the Borodin reaction or the Hunsdiecker–Borodin reaction) is a name reaction in organic chemistry whereby silver salts of carboxylic acids react with a halogen to produce an organic halide.[1] It is an example of both a decarboxylation and a halogenation reaction as the product has one fewer carbon atoms than the starting material (lost as carbon dioxide) and a halogen atom is introduced its place.[2][3] A catalytic approach has been developed.[4]
History
The reaction is named for Cläre Hunsdiecker and her husband Heinz Hunsdiecker, whose work in the 1930s[5][6] developed it into a general method.[1]
The reaction was first demonstrated by Alexander Borodin in 1861 in his reports of the preparation of methyl bromide (CH
3Br) from silver acetate (CH
3CO
2Ag).[7][8] Around the same time, Angelo Simonini, working as a student of Adolf Lieben at the University of Vienna, investigated the reactions of silver carboxylates with iodine.[2] They found that the products formed are determined by the stoichiometry within the reaction mixture. Using a carboxylate-to-iodine ratio of 1:1 leads to an alkyl iodide product, in line with Borodin's findings and the modern understanding of the Hunsdiecker reaction. However, a 2:1 ratio favours the formation of an ester product that arises from decarboxylation of one carboxylate and coupling the resulting alkyl chain with the other.[9][10]
Using a 3:2 ratio of reactants leads to the formation of a 1:1 mixture of both products.[9][10] These processes are sometimes known as the Simonini reaction rather than as modifications of the Hunsdiecker reaction.[2][3]
- 3 RCOOAg + 2 I2 → RI + RCOOR + 2 CO2 + 3 AgI
Reaction mechanism
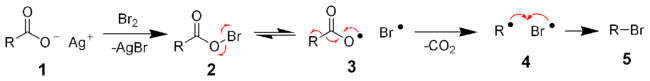
In terms of reaction mechanism, the Hunsdiecker reaction is believed to involve organic radical intermediates. The silver salt 1 reacts with bromine to form the acyl hypohalite intermediate 2. Formation of the diradical pair 3 allows for radical decarboxylation to form the diradical pair 4, which recombines to form the organic halide 5. The trend in the yield of the resulting halide is primary > secondary > tertiary.[2][3]
Variations
The reaction cannot be performed in protic solvents, as these induce decomposition of the intermediate acetyl hypohalite.[citation needed]
Other counterions than silver typically have slow reaction rates. The toxic[11] relativistic metals (mercury, thallium, and lead) are preferred: inert counterions, such as the alkali metals, have only rarely led to reported success.[12]:464
In the presence of multiple bonds, the intermediate acetyl hypohalite prefers to add to the bond, producing an α-haloester. Steric considerations suppress this tendency in α,β-unsaturated carboxylic acids, which instead polymerize (see below).[12]:468
Mercuric oxide and bromine convert 3-chlorocyclobutanecarboxylic acid to 1-bromo-3-chlorocyclobutane. This is known as Cristol-Firth modification.[13][14][15] The 1,3-dihalocyclobutanes were key precursors to propellanes.[16] The reaction has been applied to the preparation of ω-bromo esters with chain lengths between five and seventeen carbon atoms, with the preparation of methyl 5-bromovalerate published in Organic Syntheses as an exemplar.[17]
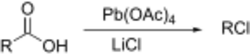
The Kochi reaction is a variation on the Hunsdiecker reaction developed by Jay Kochi that uses lead(IV) acetate and lithium chloride (lithium bromide can also be used) to effect the halogenation and decarboxylation.[18]
Reaction with α,β-unsaturated carboxylic acids
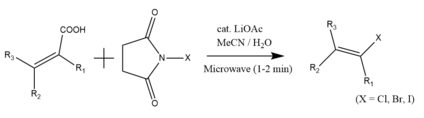
For unsaturated conmpounds, the radical conditions associated with the Hunsdiecker reaction can also induce polymerization instead of decarboxylation.[12]:468 Consequently, reactions with α,β-unsaturated carboxylic acids typically give low yield.[11] Kuang et al have found that an alternate radical halogenating agent, N-halosuccinimide, combined with a lithium acetate catalyst, gives a higher yield of β-halostyrenes. The reaction also improves in the presence of microwave irradiation, which preferentially synthesizes (E)-β-arylvinyl halides.[19]
For a green metal-free reaction, tetrabutylammonium trifluoroacetate serves as an alternative catalyst.[20] However, it only exhibits comparable yields to the original lithium acetate when performed with micellular surfactants.[19][21][22]
See also
References
- ↑ 1.0 1.1 Li, J. J. (2014-01-30). "Hunsdiecker–Borodin Reaction". Name Reactions: A Collection of Detailed Mechanisms and Synthetic Applications (5th ed.). Springer Science & Business Media. pp. 327–328. ISBN 9783319039794. https://books.google.com/books?id=HoXBBAAAQBAJ&pg=PA327.
- ↑ 2.0 2.1 2.2 2.3 Johnson, R. G.; Ingham, R. K. (1956). "The Degradation of Carboxylic Acid Salts by Means of Halogen – the Hunsdiecker Reaction". Chem. Rev. 56 (2): 219–269. doi:10.1021/cr50008a002.
- ↑ 3.0 3.1 3.2 Wilson, C. V. (1957). "The Reaction of Halogens with Silver Salts of Carboxylic Acids". Org. React. 9: 332–387. doi:10.1002/0471264180.or009.05. ISBN 0471264180.
- ↑ Wang, Zhentao; Zhu, Lin; Yin, Feng; Su, Zhongquan; Li, Zhaodong; Li, Chaozhong (2012). "Silver-Catalyzed Decarboxylative Chlorination of Aliphatic Carboxylic Acids". Journal of the American Chemical Society 134 (9): 4258–4263. doi:10.1021/ja210361z. PMID 22316183.
- ↑ Hunsdiecker, C.; E. Vogt & H. Hunsdiecker, "Method of manufacturing organic chlorine and bromine derivatives", US patent 2176181, published 1939-10-17, assigned to Hunsdiecker, C.; Vogt, E.; Hunsdiecker, H.
- ↑ Hunsdiecker, H.; Hunsdiecker, C. (1942). "Über den Abbau der Salze aliphatischer Säuren durch Brom" (in German). Chemische Berichte 75 (3): 291–297. doi:10.1002/cber.19420750309.
- ↑ Borodin, A. (1861). "Ueber Bromvaleriansäure und Brombuttersäure" (in German). Annalen der Chemie und Pharmacie 119: 121–123. doi:10.1002/jlac.18611190113. https://zenodo.org/record/1427169.
- ↑ Borodin, A. (1861). "Ueber de Monobrombaldriansäure und Monobrombuttersäure" (in German). Zeitschrift für Chemie und Pharmacie 4: 5–7. https://babel.hathitrust.org/cgi/pt?id=uc1.b3645096;view=1up;seq=39.
- ↑ 9.0 9.1 Simonini, A. (1892). "Über den Abbau der fetten Säuren zu kohlenstoffärmeren Alkoholen" (in German). Monatshefte für Chemie und verwandte Teile anderer Wissenschaften 13 (1): 320–325. doi:10.1007/BF01523646.
- ↑ 10.0 10.1 Simonini, A. (1893). "Über den Abbau der fetten Säuren zu kohlenstoffärmeren Alkoholen" (in German). Monatshefte für Chemie und verwandte Teile anderer Wissenschaften 14 (1): 81–92. doi:10.1007/BF01517859.
- ↑ 11.0 11.1 Chowdhury, Shantanu; Roy, Sujit (1997-01-01). "The First Example of a Catalytic Hunsdiecker Reaction: Synthesis of β-Halostyrenes". The Journal of Organic Chemistry 62 (1): 199–200. doi:10.1021/jo951991f. ISSN 0022-3263. PMID 11671382.
- ↑ 12.0 12.1 12.2 Tanner, Denis D.; Bunce, Nigel J. (1972). "The acyl hypohalites". in Patai, Saul. The chemistry of acyl halides. The Chemistry of Functional Groups. Bristol / London: John Wright & Sons / Interscience. doi:10.1002/9780470771273. ISBN 0-471-66936-9. https://archive.org/details/pataischemistryoffunctionalgroupsacylhalides1972/.
- ↑ Lampman, G. M.; Aumiller, J. C. (1971). "Mercury(II) oxide-modified Hunsdiecker reaction: 1-Bromo-3-chlorocyclobutane". Org. Synth. 51: 106. doi:10.15227/orgsyn.051.0106. http://www.orgsyn.org/demo.aspx?prep=cv6p0179.; Coll. Vol., 6, pp. 179
- ↑ Lampman, G. M.; Aumiller, J. C. (1971). "Bicyclo[1.1.0butane"]. Org. Synth. 51: 55. doi:10.15227/orgsyn.051.0055. http://www.orgsyn.org/demo.aspx?prep=CV6P0133.; Coll. Vol., 6, pp. 133
- ↑ Meek, J. S.; Osuga, D. T. (1963). "Bromocyclopropane". Org. Synth. 43: 9. doi:10.15227/orgsyn.043.0009. http://www.orgsyn.org/demo.aspx?prep=cv5p0126.; Coll. Vol., 5, pp. 126
- ↑ Wiberg, K. B.; Lampman, G. M.; Ciula, R. P.; Connor, D. S.; Schertler, P.; Lavanish, J. (1965). "Bicyclo[1.1.0]butane". Tetrahedron 21 (10): 2749–2769. doi:10.1016/S0040-4020(01)98361-9.
- ↑ Allen, C. F. H.; Wilson, C. V. (1946). "Methyl 5-bromovalerate (Valeric acid, δ-bromo-, methyl ester)". Org. Synth. 26: 52. doi:10.15227/orgsyn.026.0052. http://www.orgsyn.org/demo.aspx?prep=cv3p0578.; Coll. Vol., 3, pp. 578
- ↑ Kochi, J. K. (1965). "A New Method for Halodecarboxylation of Acids Using Lead(IV) Acetate". Journal of the American Chemical Society 87 (11): 2500–2502. doi:10.1021/ja01089a041.
- ↑ 19.0 19.1 Kuang, Chunxiang; Senboku, Hisanori; Tokuda, Masao (2000). "Stereoselective Synthesis of (E)-β-Arylvinyl Halides by Microwave-Induced Hunsdiecker Reaction" (in en). Synlett 2000 (10): 1439–1442. doi:10.1055/s-2000-7658. ISSN 0936-5214.
- ↑ Naskar, Dinabandhu; Chowdhury, Shantanu; Roy, Sujit (1998-02-12). "Is metal necessary in the Hunsdiecker-Borodin reaction?" (in en). Tetrahedron Letters 39 (7): 699–702. doi:10.1016/S0040-4039(97)10639-6. ISSN 0040-4039. http://www.sciencedirect.com/science/article/pii/S0040403997106396.
- ↑ Das, Jaya Prakash; Roy, Sujit (2002-11-01). "Catalytic Hunsdiecker Reaction of α,β-Unsaturated Carboxylic Acids: How Efficient Is the Catalyst?". The Journal of Organic Chemistry 67 (22): 7861–7864. doi:10.1021/jo025868h. ISSN 0022-3263. PMID 12398515.
- ↑ Rajanna, K. C.; Reddy, N. Maasi; Reddy, M. Rajender; Saiprakash, P. K. (2007-04-01). "Micellar Mediated Halodecarboxylation of α,β-Unsaturated Aliphatic and Aromatic Carboxylic Acids—A Novel Green Hunsdiecker–Borodin Reaction". Journal of Dispersion Science and Technology 28 (4): 613–616. doi:10.1080/01932690701282690. ISSN 0193-2691.
External links
 |