Chemistry:Lithium bromide
 | |
| |
| Names | |
|---|---|
| IUPAC name
Lithium bromide
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| RTECS number |
|
| UNII | |
| |
| |
| Properties | |
| LiBr | |
| Molar mass | 86.845 g/mol[1] |
| Appearance | White hygroscopic solid[1] |
| Density | 3.464 g/cm3[1] |
| Melting point | 550 °C (1,022 °F; 823 K)[1] |
| Boiling point | 1,300 °C (2,370 °F; 1,570 K)[1] |
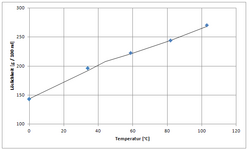
| 143 g/100 mL (0 °C) 166.7 g/100 mL (20 °C) 266 g/100 mL (100 °C)[2] | |
| Solubility | soluble in methanol, ethanol,[1] ether,[1] acetone slightly soluble in pyridine |
| −34.3·10−6 cm3/mol[3] | |
Refractive index (nD)
|
1.7843 (589 nm)[4] |
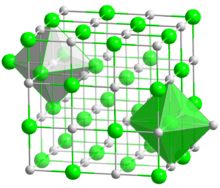
| Structure[5] | |
| Cubic, Pearson symbol cF8, No. 225 | |
| Fm3m | |
a = 0.5496 nm
| |
| Thermochemistry[6] | |
Std molar
entropy (S |
74.3 J/mol K |
Std enthalpy of
formation (ΔfH⦵298) |
−351.2 kJ/mol |
Gibbs free energy (ΔfG˚)
|
−342.0 kJ/mol |
| Hazards | |
| GHS pictograms | 
|
| GHS Signal word | Warning |
| H315, H317, H319[7] | |
| NFPA 704 (fire diamond) | |
| Flash point | Not-flammable |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose)
|
1800 mg/kg (oral, rat)[8] |
| Related compounds | |
Other anions
|
Lithium fluoride Lithium chloride Lithium iodide |
Other cations
|
Sodium bromide Potassium bromide Rubidium bromide Caesium bromide |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Lithium bromide (LiBr) is a chemical compound of lithium and bromine. Its extreme hygroscopic character makes LiBr useful as a desiccant in certain air conditioning systems.[9]
Production and properties

LiBr is prepared by treating an aqueous suspension of lithium carbonate with hydrobromic acid or by reacting lithium hydroxide with bromine.[9] It forms several crystalline hydrates, unlike the other alkali metal bromides.[10]
Lithium hydroxide and hydrobromic acid (aqueous solution of hydrogen bromide) will precipitate lithium bromide in the presence of water.
- LiOH + HBr → LiBr + H2O
Uses
A 50–60% aqueous solution of lithium bromide is used in air-conditioning systems as desiccant. It is also used in absorption chilling along with water (see absorption refrigerator). Solid LiBr is a useful reagent in organic synthesis. It is included into oxidation and hydroformylation catalysts; it is also used for deprotonation and dehydration of organic compounds containing acidic protons, and for the purification of steroids and prostaglandins.[9]
Medical applications
Lithium bromide was used as a sedative beginning in the early 1900s, but it fell into disfavor in the 1940s as newer sedatives became available and when some heart patients died after using the salt substitute lithium chloride.[11] Like lithium carbonate and lithium chloride, it was used as treatment for bipolar disorder.
Hazards
Lithium salts are psychoactive and somewhat corrosive. Heat is quickly generated when lithium bromide is dissolved into water because it has a negative enthalpy of solution.
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 Haynes, p. 4.70
- ↑ Haynes, p. 5.169
- ↑ Haynes, p. 4.128
- ↑ Haynes, p. 10.249
- ↑ Seifert, H.-J.; Dau, E. (1972). "Über die Systeme Alkalimetallbromid/Mangan(II)-bromid". Zeitschrift für Anorganische und Allgemeine Chemie 391 (3): 302–312. doi:10.1002/zaac.19723910311. Bibcode: 1972ZAACh.391..302S.
- ↑ Haynes, p. 5.25
- ↑ Lithium bromide. SIgma Aldrich
- ↑ Chambers, Michael. "ChemIDplus – 7550-35-8 – AMXOYNBUYSYVKV-UHFFFAOYSA-M – Lithium bromide – Similar structures search, synonyms, formulas, resource links, and other chemical information.". https://pubchem.ncbi.nlm.nih.gov/#tab/sidsrcname=ChemIDplus&query=7550-35-8&input_type=text.
- ↑ 9.0 9.1 9.2 Wietelmann, Ulrich and Bauer, Richard J. (2005) "Lithium and Lithium Compounds" in Ullmann's Encyclopedia of Industrial Chemistry Wiley-VCH: Weinheim. doi:10.1002/14356007.a15_393.pub2
- ↑ Holleman, Arnold Frederik; Wiberg, Egon (2001), Wiberg, Nils, ed., Inorganic Chemistry, San Diego/Berlin: Academic Press/De Gruyter, ISBN 0-12-352651-5
- ↑ "Bipolar Disorder: Treatment and Care". http://www.webmd.com/content/Article/87/99356.htm?pagenumber=1.
Cited sources
- Haynes, William M., ed (2016). CRC Handbook of Chemistry and Physics (97th ed.). CRC Press. ISBN 9781498754293.
External links
- "A PDF file from GFS Chemicals, a supplier of lithium bromide". http://www.gfschemicals.com/Search/MSDS/5035MSDS.PDF.
 |

