Chemistry:BscTx3
| BcsTx3 | |||||||
|---|---|---|---|---|---|---|---|
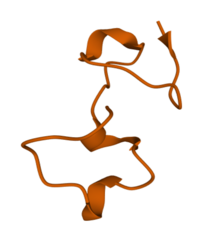 Predicted Structure of BcsTx3 from Swiss Model predictor | |||||||
| Identifiers | |||||||
| Organism | |||||||
| Symbol | N/A | ||||||
| UniProt | C0HJC4 | ||||||
| |||||||
BcsTx3, also known as Kappa-actitoxin-Bsc4a, is a neurotoxin that blocks voltage-gated potassium channel (Kv1/KCNA). It is purified from the venom of Bunodosoma caissarum.[1]
Source
This toxin is derived from the sea anemone Bunodosoma caissarum of the phylum Cnidaria[1].
Structure
Biochemical analysis of the toxin reveals that it is a small single-chain peptide consisting of 50 amino acids.[1]
Mode of Action
It selectively inhibits a variety of voltage-gated potassium channels. Electrophysiological experimentation reveals that BcsTx3 is able to block voltage-gated potassium channels Kv1.1, Kv1.2, Kv1.3, Kv1.6 as well as Drosophila Shaker IR channels. The IC50 values for these channels are 172.59 nM for Kv1.2/KCNA2, 2245.93 nM for Kv1.6/KCNA6, 1006.48 nM for human Kv1.3/KCNA3, and 94.25 nM for the Drosophila Shaker IR channels.[1] Competitive interaction with another voltage-gated potassium channel blocker, TEA, suggests that both toxins bind to the same site on the Shaker IR Kv channel.[1] It can thus be concluded that BcsTx3, like TEA, binds to the outside mouth of the pore, subsequently obstructing the flow of K+ ions.[1]
Homology
Unlike the four subtypes of previously identified sea anemone voltage-gated potassium channel toxins,[2] BcsTx3 exhibits 4 crosslinking disulphide bridges instead of 2 or 3.[1] It is therefore suggested that BcsTx3, as well as two other relatively homologous sea anemone toxins that exhibit this unique structure (nvePTx1 and msePTx1), be classified as a type 5 sea anemone Kv toxin subfamily.[1]
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 1.7 "BcsTx3 is a founder of a novel sea anemone toxin family of potassium channel blocker". The FEBS Journal 280 (19): 4839–4852. October 2013. doi:10.1111/febs.12456. PMID 23895459.
- ↑ "Discovery and characterization of cnidarian peptide toxins that affect neuronal potassium ion channels". Toxicon 54 (8): 1119–1124. December 2009. doi:10.1016/j.toxicon.2009.02.032. PMID 19269305.
 |

