Chemistry:Butoxyacetic acid
From HandWiki
| |
| Names | |
|---|---|
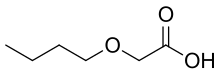
| IUPAC name
2-butoxyacetic acid
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| |
| |
| Hazards | |
| GHS pictograms | 
|
| GHS Signal word | Warning |
| H290, H314 | |
| P234, P260, P264, P264+265Script error: No such module "Preview warning".Category:GHS errors, P280, P301+330+331, P302+361+354Script error: No such module "Preview warning".Category:GHS errors, P304+340, P305+354+338Script error: No such module "Preview warning".Category:GHS errors, P316Script error: No such module "Preview warning".Category:GHS errors, P317Script error: No such module "Preview warning".Category:GHS errors, P321, P363, P390, P405, P406, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Butoxyacetic acid is an aliphatic organic chemical. It is a liquid.[2] It has the formula C6H12O3 and CAS Registry Number of 2516-93-0.[3] It is REACH registered with the EC number 677-344-8.[4] n-Butyl glycidyl ether is metabolized renally to this compound as is 2-butoxyethanol.[5][6] Methods have been developed and papers published to detect the compound in urine and blood.[7][8][9][10]
Uses
It is used as a biocide.[11]
References
- ↑ "Butoxyacetic acid" (in en). https://pubchem.ncbi.nlm.nih.gov/compound/41958#section=Safety-and-Hazards.
- ↑ "Butoxyacetic Acid 2516-93-0 | TCI AMERICA". https://www.tcichemicals.com/US/en/p/B1467.
- ↑ "AF6672500 | C6H12O3 | ChemSpider". http://www.chemspider.com/Chemical-Structure.38273.html.
- ↑ PubChem. "Butoxyacetic acid" (in en). https://pubchem.ncbi.nlm.nih.gov/compound/Butoxyacetic-acid.
- ↑ Dill, Jeffrey A.; Lee, Kyeonghee M.; Bates, Derrick J.; Anderson, David J.; Johnson, Renee E.; Chou, Billy J.; Burka, Leo T.; Roycroft, Joseph H. (1998-12-01). "Toxicokinetics of Inhaled 2-Butoxyethanol and Its Major Metabolite, 2-Butoxyacetic Acid, in F344 Rats and B6C3F1 Mice". Toxicology and Applied Pharmacology 153 (2): 227–242. doi:10.1006/taap.1998.8524. ISSN 0041-008X. PMID 9878593. https://www.sciencedirect.com/science/article/pii/S0041008X98985246.
- ↑ Eadsforth, C. V.; Hutson, D. H.; Logan, C. J.; Morrison, B. J. (1985). "The metabolism of n-butyl glycidyl ether in the rat and rabbit". Xenobiotica 15 (7): 579–89. doi:10.3109/00498258509045887. PMID 4049898.
- ↑ "Butoxy acetic acid in urine". https://www.cdc.gov/niosh/docs/2003-154/pdfs/8316.pdf.
- ↑ Udden, M. M. (2002). "In Vitro Sub-Hemolytic Effects of Butoxyacetic Acid on Human and Rat Erythrocytes". Toxicological Sciences 69 (1): 258–264. doi:10.1093/toxsci/69.1.258. PMID 12215681. https://academic.oup.com/toxsci/article-lookup/doi/10.1093/toxsci/69.1.258. Retrieved 2023-09-13.
- ↑ Rettenmeier, A. W.; Hennigs, R.; Wodarz, R. (1993-01-01). "Determination of butoxyacetic acid and N-butoxyacetyl-glutamine in urine of lacquerers exposed to 2-butoxyethanol" (in en). International Archives of Occupational and Environmental Health 65 (1): S151–S153. doi:10.1007/BF00381329. ISSN 1432-1246. PMID 8406915. https://doi.org/10.1007/BF00381329.
- ↑ BEGEROW, J; HEINRICH-RAMM, R.; ANGERER, J. (1988). "Determination of butoxyacetic acid in urine by capillary gas chromatography". Determination of Butoxyacetic Acid in Urine by Capillary Gas Chromatography 331 (8): 818–820. doi:10.1007/BF00469456. ISSN 0016-1152. https://pascal-francis.inist.fr/vibad/index.php?action=getRecordDetail&idt=7261739.
- ↑ "2-Butoxyacetic acid". https://www.biosynth.com/p/FB71946/2516-93-0-2-butoxyacetic-acid.
 |

