Chemistry:Catellani reaction
The Catellani reaction was discovered by Marta Catellani (Università degli Studi di Parma, Italy) and co-workers in 1997.[1][2] The reaction uses aryl iodides to perform bi- or tri-functionalization, including C-H functionalization of the unsubstituted ortho position(s), followed a terminating cross-coupling reaction at the ipso position. This cross-coupling cascade reaction depends on the ortho-directing transient mediator, norbornene.

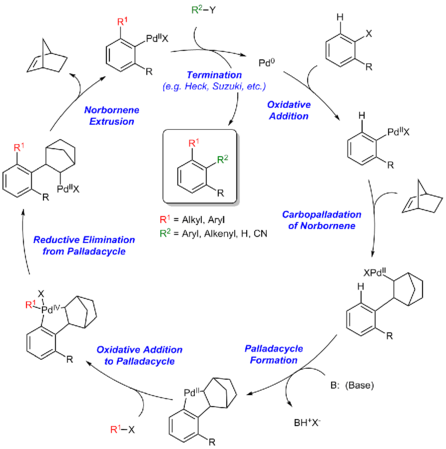
Reaction mechanism
The Catellani reaction is catalyzed by palladium and norbornene, although in most cases superstochiometric amounts of norbornene are used to allow the reaction to proceed at a reasonable rate.[4] The generally accepted reaction mechanism, as outlined below, is intricate and believed to proceed via a series of Pd(0), Pd(II), and Pd(IV) intermediates, although an alternative bimetallic mechanism that avoids the formation of Pd(IV) has also been suggested.[5]
Initially, Pd(0) oxidatively adds into the C–X bond of the aryl halide. Subsequently, the arylpalladium(II) species undergoes carbopalladation with the norbornene. The structure of the norbornylpalladium intermediate does not allow for β-hydride elimination at either of the β-positions due to Bredt's Rule for the bridgehead β-hydrogen and the trans-configuration between palladium and other β-hydrogen.[6] Thereafter, the Pd(II) species undergoes electrophilic cyclopalladation at the ortho position of the aryl group. Subsequently, the palladacyclic intermediate undergoes a second oxidation addition with the alkyl halide coupling partner to form a Pd(IV) intermediate, which undergoes reductive elimination to forge the first C–C bond of the product. After β-carbon elimination of norbornene, the resultant Pd(II) species then undergoes a second C–C bond forming step via a Heck reaction or cross coupling with an organoboron reagent to afford the final organic product and close the catalytic cycle.[7]
Steps of the Catellani reaction:
- Oxidative addition
- Carbopalladation of norbornene
- Palladacycle formation
- Oxidative addition to palladacycle
- Reductive elimination from palladacycle
- Norbornene extrusion
- Termination via Heck reaction, Suzuki reaction, etc.
Ortho and ipso cross-coupling partners
The Catellani reaction facilitates a variety of C—C and C—N bond-forming reactions at the ortho position. These include alkylation from alkyl halides,[1] arylation from aryl bromides,[8] amination from benzyloxyamines,[9][10][11][12] acylation from anhydrides.[13][14] Likewise in the case of terminating ipso coupling partners with Heck-type termination with olefins,[1] Suzuki-type reaction with boronic esters,[9] borylation with bis(pinacolato)diboron,[10][15] protonation with i-PrOH,[11] decarboxylative alkynylation with alkynyl carboxylic acids.[12]
Uses
With tethered cross-coupling partners, Lautens, Malacria, and Catellani used this reaction to construct a variety of fused ring systems since 2000.[7] The Catellani reaction has been used as a key step for the total synthesis (+)-linoxepin,[16] rhazinal,[17] aspidospermidine,[18] and (±)-goniomitine.[18]
References
- ↑ 1.0 1.1 1.2 Catellani, Marta; Frignani, Franco; Rangoni, Armando (1997-02-03). "A Complex Catalytic Cycle Leading to a Regioselective Synthesis of o,o′-Disubstituted Vinylarenes" (in en). Angewandte Chemie International Edition in English 36 (1–2): 119–122. doi:10.1002/anie.199701191. ISSN 1521-3773.
- ↑ Catellani (1997). "Regioselektive Synthese o,o′-disubstituierter Vinylarene über einen komplexen Katalysecyclus". Angewandte Chemie 109 (1–2): 142–145. doi:10.1002/ange.19971090146.
- ↑ Martins (2010). "Synthesis in the Key of Catellani: Norbornene-Mediated ortho C–H Functionalization". C-H Activation. Topics in Current Chemistry. 292. pp. 1–33. doi:10.1007/128_2009_13. ISBN 978-3-642-12355-9.
- ↑ Catellani, Marta; Motti, Elena; Della Ca’, Nicola (2008-11-18). "Catalytic Sequential Reactions Involving Palladacycle-Directed Aryl Coupling Steps". Accounts of Chemical Research 41 (11): 1512–1522. doi:10.1021/ar800040u. ISSN 0001-4842. PMID 18680317.
- ↑ Cárdenas, Diego J.; Martín-Matute, Belén; Echavarren, Antonio M. (2006). "Aryl Transfer between Pd(II) Centers or Pd(IV) Intermediates in Pd-Catalyzed Domino Reactions". Journal of the American Chemical Society 128 (15): 5033–5040. doi:10.1021/ja056661j. PMID 16608337.
- ↑ Martins (2010). "Synthesis in the Key of Catellani: Norbornene-Mediated ortho C–H Functionalization". C-H Activation. Topics in Current Chemistry. 292. pp. 1–33. doi:10.1007/128_2009_13. ISBN 978-3-642-12355-9.
- ↑ 7.0 7.1 Ye, Juntao; Lautens, Mark (2015). "Palladium-catalysed norbornene-mediated C–H functionalization of arenes". Nature Chemistry 7 (11): 863–870. doi:10.1038/nchem.2372. PMID 26492005. Bibcode: 2015NatCh...7..863Y.
- ↑ Faccini, Fiorenza; Motti, Elena; Catellani, Marta (2004-01-01). "A New Reaction Sequence Involving Palladium-Catalyzed Unsymmetrical Aryl Coupling". Journal of the American Chemical Society 126 (1): 78–79. doi:10.1021/ja039043g. ISSN 0002-7863. PMID 14709068.
- ↑ 9.0 9.1 Ye, Changqing; Zhu, Hui; Chen, Zhiyuan (2014-09-19). "Synthesis of Biaryl Tertiary Amines through Pd/Norbornene Joint Catalysis in a Remote C–H Amination/Suzuki Coupling Reaction". The Journal of Organic Chemistry 79 (18): 8900–8905. doi:10.1021/jo501544h. ISSN 0022-3263. PMID 25171687.
- ↑ 10.0 10.1 Shi, Hang; Babinski, David J.; Ritter, Tobias (2015-03-25). "Modular C–H Functionalization Cascade of Aryl Iodides". Journal of the American Chemical Society 137 (11): 3775–3778. doi:10.1021/jacs.5b01082. ISSN 0002-7863. PMID 25763682.
- ↑ 11.0 11.1 Dong, Zhe; Dong, Guangbin (2013-12-11). "Ortho vs Ipso: Site-Selective Pd and Norbornene-Catalyzed Arene C–H Amination Using Aryl Halides". Journal of the American Chemical Society 135 (49): 18350–18353. doi:10.1021/ja410823e. ISSN 0002-7863. PMID 24256439.
- ↑ 12.0 12.1 Sun, Fenggang; Gu, Zhenhua (2015-05-01). "Decarboxylative Alkynyl Termination of Palladium-Catalyzed Catellani Reaction: A Facile Synthesis of α-Alkynyl Anilines via Ortho C–H Amination and Alkynylation". Organic Letters 17 (9): 2222–2225. doi:10.1021/acs.orglett.5b00830. ISSN 1523-7060. PMID 25899570.
- ↑ Huang, Yunze; Zhu, Rui; Zhao, Kun; Gu, Zhenhua (2015-10-19). "Palladium-Catalyzed Catellani ortho-Acylation Reaction: An Efficient and Regiospecific Synthesis of Diaryl Ketones" (in en). Angewandte Chemie International Edition 54 (43): 12669–12672. doi:10.1002/anie.201506446. ISSN 1521-3773. PMID 26331234.
- ↑ Zhou, Ping-Xin; Ye, Yu-Ying; Liu, Ce; Zhao, Lian-Biao; Hou, Jian-Ye; Chen, Dao-Qian; Tang, Qian; Wang, An-Qi et al. (2015-08-07). "Palladium-Catalyzed Acylation/Alkenylation of Aryl Iodide: A Domino Approach Based on the Catellani–Lautens Reaction". ACS Catalysis 5 (8): 4927–4931. doi:10.1021/acscatal.5b00516.
- ↑ Sui, Xianwei; Grigolo, Thiago A.; O’Connor, Colin J.; Smith, Joel M. (2019-11-15). "Ortho/Ipso Alkylborylation of Aryl Iodides". Organic Letters 21 (22): 9251–9255. doi:10.1021/acs.orglett.9b03674. ISSN 1523-7060. PMID 31696718. https://doi.org/10.1021/acs.orglett.9b03674.
- ↑ Weinstabl (16 Apr 2013). "Total Synthesis of (+)-Linoxepin by Utilizing the Catellani Reaction". Angewandte Chemie International Edition 52 (20): 5305–5308. doi:10.1002/anie.201302327. PMID 23592590.
- ↑ Sui (June 2013). "Pd-Catalyzed Chemoselective Catellani Ortho-Arylation of Iodopyrroles: Rapid Total Synthesis of Rhazinal". J. Am. Chem. Soc. 135 (25): 9318–9321. doi:10.1021/ja404494u. PMID 23758183.
- ↑ 18.0 18.1 Jiao, Lei; Herdtweck, Eberhardt; Bach, Thorsten (2012-09-05). "Pd(II)-Catalyzed Regioselective 2-Alkylation of Indoles via a Norbornene-Mediated C–H Activation: Mechanism and Applications". Journal of the American Chemical Society 134 (35): 14563–14572. doi:10.1021/ja3058138. ISSN 0002-7863. PMID 22913367.
External links
 |

