Chemistry:Copper-catalyzed allylic substitution
Copper-catalyzed allylic substitutions are chemical reactions with unique regioselectivity compared to other transition-metal-catalyzed allylic substitutions such as the Tsuji-Trost reaction. They involve copper catalysts and "hard" carbon nucleophiles. The mechanism of copper-catalyzed allylic substitutions involves the coordination of copper to the olefin, oxidative addition and reductive elimination. Enantioselective versions of these reactions have been used in the synthesis of complex molecules, such as (R)-(-)-sporochnol and (S)-(-)-zearalenone.
Features
Copper-catalyzed allylic substitutions are characterized by their unique regioselectivity compared to other transition-metal-catalyzed allylic substitutions, the most well-known being the palladium-catalyzed Tsuji-Trost reaction.[1] The distinct mechanism of copper-catalyzed allylic substitutions has been known to provide high regioselectivity of the γ substituted product, compared to the α substituted isomer.[1] The copper catalyst used can be symmetrical with two identical R groups, or with two different ligands. These reactions typically utilize “hard” carbon nucleophiles such as Grignard, diorganozinc, organolithium, and trialkyl aluminum reagents.[1] This contrasts palladium-catalyzed allylic substitutions which involve “soft” nucleophiles..[1]
Mechanism
The catalytic cycle begins with coordination of the Cu(I) species to the olefin, followed by oxidative addition at the γ position and an allylic shift to displace the leaving group.[2] This generates a Cu(III) allyl complex intermediate.[2] Finally, reductive elimination yields the final product and regenerates Cu(I).[2] A Cu(III) intermediate has not been confirmed by isolation from allylic substitutions, but Cu(III) intermediates have been isolated before, thus providing credence to the proposed mechanism.[2] If reductive elimination does not occur fast enough, the γ allyl complex can isomerize to the α allyl complex and yield the α substituted isomer as a byproduct. This side pathway can be prevented by using electron withdrawing ligands on copper, typically a cyanide or halide ligand, which promote reductive elimination. [3]
Asymmetric copper-catalyzed allylic substitution
Mechanistically, oxidative addition is the step that determines which enantiomer is formed.[3] Chiral ligands on the metal center along with low temperatures are the general tactics employed to produce an enantiopure product.[4] In particular, the careful pairing of ligand classes with the type of nucleophile has proven to be essential. With Grignard reagents, ferrocenyl thiolate,[5][6] phosphorus,[7] and NHC ligands[8] are typically used. There have also been several methods developed using diorganozinc nucleophiles coupled with phosphorus,[9][10] amine,[11] peptide,[12] and NHC[13] ligands. The scope of organoaluminium nucleophiles is comparatively smaller, but there have been a couple examples using NHC ligands.[14] There is a need for more studies to better understand the mechanism of stereoinduction to expand the known set of reactions to encompass a larger overall substrate scope and to potentially allow for enantioselectivity at room temperature.[4]
Applications in natural product synthesis
There have been several enantioselective versions of this reaction developed, and even employed in synthesis of complex molecules. Hoyveda's synthesis of (R)-(-)-sporochnol included an asymmetric copper-catalyzed allylic substitution with an organozinc nucleophile and peptide ligand.[11]
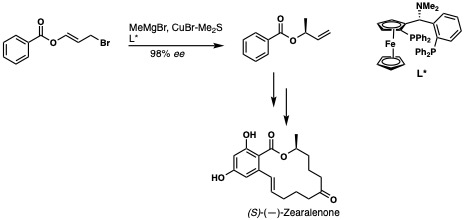
A TaniaPHOS ligand, a ferrocenylphosphine, is used with a methyl Grignard nucleophile to form an allylic stereocenter towards the total synthesis of (S)-(-)-Zearalenone[15]
Allylic substitutions are one class of the several types of reactions carried out by organocuprate reagents.
References
- ↑ 1.0 1.1 1.2 1.3 Hartwig, John Frederick (2010). Organotransition metal chemistry: from bonding to catalysis (1 ed.). Sausalito (Calif.): University science books. ISBN 978-1891389535.
- ↑ 2.0 2.1 2.2 2.3 Yoshikai, Naohiko; Nakamura, Eiichi (11 April 2012). "Mechanisms of Nucleophilic Organocopper(I) Reactions". Chemical Reviews 112 (4): 2339–2372. doi:10.1021/cr200241f. PMID 22111574.
- ↑ 3.0 3.1 Alexakis, Alexandre; Malan, Christophe; Lea, Louise; Tissot-Croset, Karine; Polet, Damien; Falciola, Caroline (28 March 2006). "The Copper-Catalyzed Asymmetric Allylic Substitution". CHIMIA 60 (3): 124. doi:10.2533/000942906777674994.
- ↑ 4.0 4.1 Cotton, Hanna K.; Norinder, Jakob; Bäckvall, Jan-E. (12 June 2006). "Screening of ligands in the asymmetric metallocenethiolatocopper(I)-catalyzed allylic substitution with Grignard reagents". Tetrahedron 62 (24): 5632–5640. doi:10.1016/j.tet.2006.03.100.
- ↑ Alexakis, Alexandre; Croset, Karine (1 November 2002). "Tandem Copper-Catalyzed Enantioselective Allylation−Metathesis". Organic Letters 4 (23): 4147–4149. doi:10.1021/ol0269244. PMID 12423108.
- ↑ Alexakis, Alexandre; Croset, Karine (2002-11-01). "Tandem Copper-Catalyzed Enantioselective Allylation−Metathesis" (in en). Organic Letters 4 (23): 4147–4149. doi:10.1021/ol0269244. ISSN 1523-7060. PMID 12423108. https://pubs.acs.org/doi/10.1021/ol0269244.
- ↑ Tominaga, Satoshi; Oi, Yukinao; Kato, Toshio; An, Duk Keun; Okamoto, Sentaro (12 July 2004). "γ-Selective allylic substitution reaction with Grignard reagents catalyzed by copper N-heterocyclic carbene complexes and its application to enantioselective synthesis". Tetrahedron Letters 45 (29): 5585–5588. doi:10.1016/j.tetlet.2004.05.135.
- ↑ van Zijl, Anthoni W.; Arnold, Leggy A.; Minnaard, Adriaan J.; Feringa, Ben L. (March 2004). "Highly Enantioselective Copper-Catalyzed Allylic Alkylation with Phosphoramidite Ligands". Advanced Synthesis & Catalysis 346 (4): 413–420. doi:10.1002/adsc.200303207. https://research.rug.nl/en/publications/c4df9a21-c369-4131-9f56-7236955a67b9.
- ↑ Tissot-Croset, Karine; Polet, Damien; Alexakis, Alexandre (26 April 2004). "A Highly Effective Phosphoramidite Ligand for Asymmetric Allylic Substitution". Angewandte Chemie International Edition 43 (18): 2426–2428. doi:10.1002/anie.200353744. PMID 15114581.
- ↑ Goldsmith, Paul J.; Teat, Simon J.; Woodward, Simon (8 April 2005). "Enantioselective Preparation of ?,?-Disubstituted ?-Methylenepropionates by MAO Promotion of the Zinc Schlenk Equilibrium". Angewandte Chemie 117 (15): 2275–2277. doi:10.1002/ange.200463028. Bibcode: 2005AngCh.117.2275G.
- ↑ 11.0 11.1 Luchaco-Cullis, Courtney A.; Mizutani, Hirotake; Murphy, Kerry E.; Hoveyda, Amir H. (17 April 2001). "Modular Pyridinyl Peptide Ligands in Asymmetric Catalysis: Enantioselective Synthesis of Quaternary Carbon Atoms Through Copper-Catalyzed Allylic Substitutions". Angewandte Chemie International Edition 40 (8): 1456–1460. doi:10.1002/1521-3773(20010417)40:8<1456::AID-ANIE1456>3.0.CO;2-T.
- ↑ Larsen, Andrew O.; Leu, Wenhao; Oberhuber, Christina Nieto; Campbell, John E.; Hoveyda, Amir H. (1 September 2004). "Bidentate NHC-Based Chiral Ligands for Efficient Cu-Catalyzed Enantioselective Allylic Alkylations: Structure and Activity of an Air-Stable Chiral Cu Complex". Journal of the American Chemical Society 126 (36): 11130–11131. doi:10.1021/ja046245j. PMID 15355076.
- ↑ Geurts, Koen; Fletcher, Stephen P.; Zijl, Anthoni W. van; Minnaard, Adriaan J.; Feringa, Ben L. (2008-01-01). "Copper-catalyzed asymmetric allylic substitution reactions with organozinc and Grignard reagents" (in en). Pure and Applied Chemistry 80 (5): 1025–1037. doi:10.1351/pac200880051025. ISSN 1365-3075. https://www.degruyter.com/document/doi/10.1351/pac200880051025/html.
- ↑ Lee, Yunmi; Akiyama, Katsuhiro; Gillingham, Dennis G.; Brown, M. Kevin; Hoveyda, Amir H. (1 January 2008). "Highly Site- and Enantioselective Cu-Catalyzed Allylic Alkylation Reactions with Easily Accessible Vinylaluminum Reagents". Journal of the American Chemical Society 130 (2): 446–447. doi:10.1021/ja0782192. PMID 18088127.
- ↑ Baggelaar, Marc P.; Huang, Yange; Feringa, Ben L.; Dekker, Frank J.; Minnaard, Adriaan J. (2013-09-01). "Catalytic asymmetric total synthesis of (S)-(−)-zearalenone, a novel lipoxygenase inhibitor" (in en). Bioorganic & Medicinal Chemistry 21 (17): 5271–5274. doi:10.1016/j.bmc.2013.06.024. ISSN 0968-0896. PMID 23867388. https://www.sciencedirect.com/science/article/pii/S0968089613005555.
 |