Chemistry:Doyle–Kirmse reaction
The Doyle–Kirmse reaction is an organic reaction in which a metal carbene reacts with an allyl compound with transposition of the alkene and transfer of the electronegative group from the allyl onto the carbene carbon.
As originally developed, an allyl sulfide reacts with trimethylsilyldiazomethane to form the homoallyl sulfide compound.[1] The reaction was first reported by Wolfgang Kirmse (de) in 1968[2] and modified by Michael P. Doyle in 1981.[3]
The Kirmse protocol required a copper salt. The reaction mechanism involves nucleophilic addition of the sulfur to the metal carbene formed from the diazoalkane followed by a Stevens-like rearrangement.
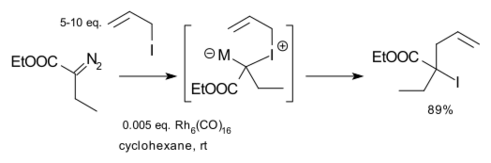
Doyle expanded the scope of the reaction to include other diazo compounds, such as ethyl diazoacetate, other allyl compounds, such as allyl amines and allyl halides, and use of with rhodium catalysts, such as hexadecacarbonylhexarhodium. An example is the reaction of ethyl diazoacetate with allyl iodide:
The reaction can also be catalyzed by iron,[4] palladium[5] silver,[6] and nickel.[7] Modifications using other carbenes are reported e.g. (2-furyl)carbenoids.[8]
The reaction is not strictly limited to allyl compounds. Propargyl-sulfide substrates give allene products[6][9] and conversely allenyl-sulfide substrates give homopropargyl products.[10]
Using metal catalysts that have chiral ligands leads to stereoselectivity of the newly-formed carbon–carbon bond.[7][10]
References
- ↑ Mundy, Bradford P.; Ellerd, Michael G.; Favaloro, Frank G. (21 April 2005). Name reactions and reagents in organic synthesis. ISBN 978-0471228547.
- ↑ Kirmse, W.; Kapps, M. (1968). "Reaktionen des Diazomethans mit Diallylsulfid und Allyläthern unter Kupfersalz-Katalyse" (in de). Chemische Berichte 101 (3): 994–1003. doi:10.1002/cber.19681010333.
- ↑ Doyle, Michael P.; Tamblyn, William H.; Bagheri, Vahid (1981). "Highly effective catalytic methods for ylide generation from diazo compounds. Mechanism of the rhodium- and copper-catalyzed reactions with allylic compounds". J. Org. Chem. 46 (25): 5094–5102. doi:10.1021/jo00338a008.
- ↑ Carter, David S.; Van Vranken, David L. (2000). "Iron-Catalyzed Doyle−Kirmse Reaction of Allyl Sulfides with (Trimethylsilyl)diazomethane". Org. Lett. 2 (9): 1303–1305. doi:10.1021/ol005740r. PMID 10810733.
- ↑ Greenman, Kevin L.; Carter, David S.; Van Vranken, David L. (2001). "Palladium-catalyzed insertion reactions of trimethylsilyldiazomethane". Tetrahedron 57 (24): 5219–5225. doi:10.1016/S0040-4020(01)00363-5.
- ↑ 6.0 6.1 Davies, Paul W.; Albrecht, Sébastien J.-C.; Assanelli, Giulio (2009). "Silver-catalysed Doyle–Kirmse reaction of allyl and propargyl sulfides". Org. Biomol. Chem. 7 (7): 1276–1279. doi:10.1039/B822584B. PMID 19300808.
- ↑ 7.0 7.1 Lin, Xiaobin; Tang, Yu; Yang, Wei; Tan, Fei; Lin, Lili; Liu, Xiaohua; Feng, Xiaoming (2018). "Chiral Nickel(II) Complex Catalyzed Enantioselective Doyle–Kirmse Reaction of α-Diazo Pyrazoleamides". J. Am. Chem. Soc. 140 (9): 3299–3305. doi:10.1021/jacs.7b12486. PMID 29444400.
- ↑ Kato, Yumiko; Miki, Koji; Nishino, Fumiaki; Ohe, Kouichi; Uemura, Sakae (2003). "Doyle–Kirmse Reaction of Allylic Sulfides with Diazoalkane-Free (2-Furyl)carbenoid Transfer". Organic Letters 5 (15): 2619–2621. doi:10.1021/ol034731q. PMID 12868873.
- ↑ Prabharasuth, Rosalind; Van Vranken, David L. (2001). "Iron-Catalyzed Reaction of Propargyl Sulfides and Trimethylsilyldiazomethane". Journal of Organic Chemistry 66 (15): 5256–5258. doi:10.1021/jo010247u. PMID 11463283.
- ↑ 10.0 10.1 Wang, Kang; Li, Shu-Sen; Wang, Jianbo (2022). "Cu(I)/Chiral Bisoxazoline-Catalyzed Enantioselective Doyle–Kirmse Reaction of Allenyl Sulfides with α-Diazoesters". Chemistry: A European Journal 28 (21): e202200170. doi:10.1002/chem.202200170. PMID 35188308.
 |