Chemistry:Fischer–Hepp rearrangement
| Fischer-Hepp rearrangement | |
|---|---|
| Named after | Otto Fischer Eduard Hepp |
| Reaction type | Rearrangement reaction |
| Identifiers | |
| RSC ontology ID | RXNO:0000095 |
In organic chemistry, the Fischer–Hepp rearrangement is a rearrangement reaction in which an aromatic N-nitroso (–N=O) or secondary nitrosamine (>N–N=O) converts to a carbon nitroso compound:[1][2]
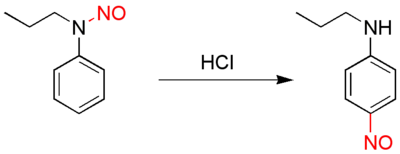
This organic reaction was first described by the German chemist Otto Philipp Fischer (1852–1932) and Eduard Hepp (June 11, 1851 – June 18, 1917) [3] in 1886, and is of importance because para-NO secondary anilines cannot be prepared in a direct reaction.
The rearrangement reaction takes place by reacting the nitrosamine precursor with hydrochloric acid. The chemical yield is generally good under these conditions, but often much poorer if a different acid is used.[4] The exact reaction mechanism is unknown but the chloride counterion is likely not relevant, except in a competing decomposition reaction. There is evidence suggesting an intramolecular reaction, similar to that seen in the Bamberger rearrangement. Nitrosation follows the classic patterns of electrophilic aromatic substitution (for example, a meta nitro group inhibits the reaction), although substitution ortho to the amine is virtually unknown. The final step, in which a proton eliminates from the Wheland intermediate, appears to be rate-limiting, and the rearrangement is also suppressed in excessive (e.g. >10M sulfuric) acid.[5]
See also
- Friedel–Crafts alkylation-like reactions:
- Hofmann-Martius rearrangement
- Fries rearrangement
References
- ↑ Fischer, Otto; Hepp, Eduard (July–December 1886). "Zur Kenntniss der Nitrosamine" (in en). Berichte der Deutschen Chemischen Gesellschaft zu Berlin 19 (2): 2991–2995. doi:10.1002/cber.188601902297. Zenodo 1425449. Gallica ark:/12148/bpt6k907075/f473.item. ISSN 0365-9496. https://zenodo.org/record/1425449.
- ↑ M B Smith, J March. March's Advanced Organic Chemistry (Wiley, 2001) (ISBN 0-471-58589-0) / Michael B., Smith (2013). "11.6.2.2 Groups Cleaving from Nitrogen; Reaction 11-29: Migration of the Nitroso Group: The Fischer–Hepp Rearrangement". March's Advanced Organic Chemistry - Reactions, Mechanisms, and Structure (7th ed.). Hoboken, New Jersey: John Wiley & Sons, Inc.. p. 639. ISBN 978-0-470-46259-1. https://app.knovel.com/hotlink/pdf/id:kt011AYKO1/marchs-advanced-organic/aromatic-s-groups-cleaving.
- ↑ Pötsch, Winfried R.; Fischer, Annelore; Müller, Wolfgang (1988–1989). Lexikon bedeutender Chemiker. With the collaboration of Heinz Cassebaum. Thun & Frankfurt: Verlag Harri Deutsch / VEB Bibliographisches Institut Leipzig. pp. 148, 197. ISBN 3-8171-1055-3.
- ↑ Smith, Michael B. (2020). March's Organic Chemistry (8th ed.). Wiley. pp. 678–679.
- ↑ Williams, D. L. H. (1988). Nitrosation. Cambridge, UK: Cambridge University. pp. 115–125. ISBN 0-521-26796-X.
Sources
- Andraos, John (2000–2012). "Named Things in Chemical Industry". https://careerchem.com/NAMED/Industry.pdf.
 |
