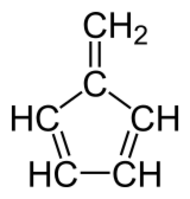
Chemistry:Fulvenes
Fulvenes are the class of hydrocarbon obtained by formally cross-conjugating one ring and methylidene through a common exocyclic double bond.[1][2]
The name is derived from fulvene, which has one pentagonal ring. Other examples include methylenecyclopropene (triafulvene) and heptafulvene.
Subclasses
Several types of fulvenes are defined.[3] They are:
- triafulvene
- pentafulvene
- heptafulvene
- nonafulvene
Preparation
Fulvenes are readily prepared by the condensation of cyclopentadiene and aldehydes and ketones:
- C5H6 + R2C=O → C4H4C=CR2 + H2O
Thiele is credited with discovering this reaction.[4][5]
Modern synthesis of fulvenes employ buffer systems.[6][7]
Ligand in organometallic chemistry
Fulvenes are common ligands and ligand precursors in organometallic chemistry.[8] 2,3,4,5-Tetramethylfulvene, abbreviated Me4Fv, results from the deprotonation of cationic pentamethylcyclopentadienyl complexes.[9] Some Me4Fv complexes are called tuck-in complexes.

References
- ↑ Agranat, Israel (2012), "Ground-State Versus Excited-State Polarity of Triafulvenes: A Study of Solvent Effects on Molecular Electronic Spectra", The Jerusalem Symposia on Quantum Chemistry and Biochemistry 8: 573–583, doi:10.1007/978-94-010-1837-1_36
- ↑ Neuenschwander, Markus (1986), "Synthetic and NMR spectroscopic investigations of fulvenes and fulvalenes", Pure Appl. Chem. 58 (1): 55–66, doi:10.1351/pac198658010055, http://pac.iupac.org/publications/pac/pdf/1986/pdf/5801x0055.pdf
- ↑ IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "Fulvenes". doi:10.1351/goldbook.F02550
- ↑ Thiele, J. (1900). "Ueber Ketonreactionen bei dem Cyclopentadiën". Chemische Berichte 33: 666–673. doi:10.1002/cber.190003301113. https://zenodo.org/record/1425954.
- ↑ Hafner, K.; Vöpel, K. H.; Ploss, G.; König, C. (1967). "6-(Dimethylamino)Fulvene". Organic Syntheses 47: 52. doi:10.15227/orgsyn.047.0052.
- ↑ Coşkun, Necdet; Erden, Ihsan (2011-11-11). "An efficient catalytic method for fulvene synthesis". Tetrahedron 67 (45): 8607–8614. doi:10.1016/j.tet.2011.09.036. ISSN 0040-4020. PMID 22021940.
- ↑ Sieverding, Paul; Osterbrink, Johanna; Besson, Claire; Kögerler, Paul (2019-01-18). "Kinetics and mechanism of pyrrolidine buffer-catalyzed fulvene formation". J. Org. Chem. 84 (2): 486–494. doi:10.1021/acs.joc.8b01660. ISSN 0022-3263. PMID 30540466.
- ↑ Strohfeldt, Katja; Tacke, Matthias (2008). "Bioorganometallic fulvene-derived titanocene anti-cancer drugs". Chemical Society Reviews 37 (6): 1174–87. doi:10.1039/B707310K. PMID 18497930.
- ↑ Kreindlin, A. Z.; Rybinskaya, M. A. (2004). "Cationic and Neutral Transition Metal Complexes with a Tetramethylfulvene or Trimethylallyldiene Ligand". Russian Chemical Reviews 73 (5): 417–432. doi:10.1070/RC2004v073n05ABEH000842. Bibcode: 2004RuCRv..73..417K.
 |