Chemistry:Gallium 68 PSMA-11
 | |
 | |
| Clinical data | |
|---|---|
| Other names | GALLIUM GA-68 GOZETOTIDE; (68)Ga Labeled Glu-NH-CO-NH-Lys(ahx)-hbed-CC |
| AHFS/Drugs.com | |
| Routes of administration | intravenous (IV)[1] |
| Pharmacokinetic data | |
| Excretion | Urine[1] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| Chemical and physical data | |
| 3D model (JSmol) | |
| |
| |
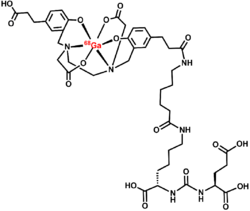
Gallium 68 PSMA-11, or Ga 68 PSMA-11, is a radiopharmaceutical made of 68Ga conjugated to prostate-specific membrane antigen (PSMA) targeting ligand, Glu-Urea-Lys(Ahx)-HBED-CC, used for imaging prostate cancer by positron emission tomography (PET).[2] The PSMA targeting ligand specifically directs the radiolabeled imaging agent towards the prostate cancerous lesions in men. [3] This is the first drug approved by USFDA as a PET imaging agent.[3]
Development
Initially Gallium (68Ga) chloride solution injections used for radiolabelling,[4] in 2019 European Pharmacopoeia mentions Gallium (68Ga) DOTATOC injection for radiolabelling and PET imaging.[5]
Ga 68 PSMA-11 is co-developed by University of California, Los Angeles and University of California, San Francisco, they conducted phase 3 clinical trial.[6] On 1st December 2020, the drug was first approved by USFDA for PET imaging.[3]
Mechanism of action
PSMA-11, Glu-Urea-Lys(Ahx)-HBED-CC of Ga 68 PSMA-11, directs and binds with prostate-specific membrane antigen (PSMA). This usually binds to cells that overexpress PSMA, such as the malignant prostate cancer cells. The radioactive Isotope of Gallium, Ga-68 is responsible for emitting β+ radiations and X-rays. This helps in recording images by positron emission tomography (PET) and CT scan.[1]
Usage
Ga 68 PSMA-11 injections are used for PET imaging of prostate-specific membrane antigen (PSMA) positive lesions in males with prostate cancer. It can be given for the patients with suspected metastasis, and the candidates with initial definitive therapy.[1]
References
- ↑ 1.0 1.1 1.2 1.3 "Highlights of Prescribing Information: Gallium Ga 68 PSMA-11 Injection, for intravenous use". FDA. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/212642s000lbl.pdf. "These highlights do not include all the information needed to use Gallium Ga 68 PSMA-11 Injection safely and effectively. See full prescribing information for Gallium Ga 68 PSMA-11 Injection."
- ↑ "gallium Ga 68 gozetotide" (in en). 2 February 2011. https://www.cancer.gov/publications/dictionaries/cancer-drug/def/gallium-ga-68-gozetotide?redirect=true.
- ↑ 3.0 3.1 3.2 Office of the Commissioner (2 December 2020). "FDA Approves First PSMA-Targeted PET Imaging Drug for Men with Prostate Cancer" (in en). https://www.fda.gov/news-events/press-announcements/fda-approves-first-psma-targeted-pet-imaging-drug-men-prostate-cancer.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ↑ "Gallium (68Ga) Chloride Solution for Radiolabelling". European Pharmacopoeia (9th ed.). Stuttgart. 2018. p. 1148. ISBN 978-3-7692-6816-4.
- ↑ "Gallium (68Ga) DOTATOC injection". European Pharmacopoeia (10th ed.). Stuttgart. 2019. p. 1208. ISBN 978-3-7692-7453-0.
- ↑ "68Ga-PSMA-11 NDA Approval: A Novel and Successful Academic Partnership". Journal of Nuclear Medicine 62 (2): 149–155. February 2021. doi:10.2967/jnumed.120.260455. PMID 33443068.
