Chemistry:Guar hydroxypropyltrimonium chloride
From HandWiki
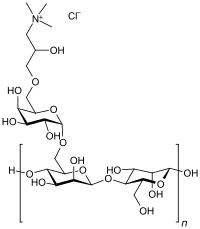
| |
| Names | |
|---|---|
| Other names
Guar, 2-hydroxy-3-trimethylammoniopropyl ether, chloride
| |
| Identifiers | |
| RTECS number |
|
| UNII | |
| Properties | |
| Density | 1.3 |
| Hazards | |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose)
|
1.25g/kg rat[1] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
Guar hydroxypropyltrimonium chloride is an organic compound that is a water-soluble quaternary ammonium derivative of guar gum. It gives conditioning properties to shampoos and after-shampoo hair care products. The effects of the cationic charge density, guar concentration in aqueous solution, and treatment time on bleached European hair have been studied. A mechanical testing method has been successfully applied to determine the efficacy of cationic guars to improve the ease of combing. The results were confirmed in a shampoo formulation on both virgin and bleached hair.[2]
References
- ↑ Chambers, Michael. "ChemIDplus - 65497-29-2 - Guar gum, 2-hydroxy-3-(trimethylammonio)propyl ether, chloride - Similar structures search, synonyms, formulas, resource links, and other chemical information." (in en). https://chem.nlm.nih.gov/chemidplus/rn/65497-29-2.
- ↑ Stephen R. Bell, "Cleansing compositions containing conditioning agents and refined agricultural grains", US patent 5817608, issued 1998-10-06, assigned to Brimms Inc.
 |

