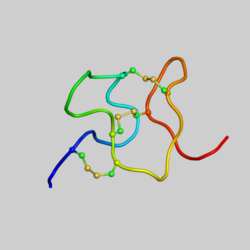
Chemistry:Heteropodatoxin
Heteropodatoxins are peptide toxins from the venom of the giant crab spider Heteropoda venatoria, which block Kv4.2 voltage-gated potassium channels.
Sources
Heteropodatoxins are purified from the venom of the giant crab spider, Heteropoda venatoria.[2]
Chemistry
Heteropodatoxins contain an Inhibitor Cystine Knot (ICK) motif, which consist of a compact disulfide-bonded core, from which four loops emerge.[1] There are three different heteropodatoxins:[2]
- heteropodatoxin-1, also known as Toxin AU3/KJ5 or HpTx1
- heteropodatoxin-2, also known as Toxin KJ6 or HpTx2
- heteropodatoxin-3, also known as Toxin AU5C/KJ7 or HpTx3
These three toxins are structurally similar peptides of 29-32 amino acids.[2] They show sequence similarity to Hanatoxins, which can be isolated from the venom of the Chilean rose tarantula Grammostola rosea.[2]
Target
Heteropodatoxins block A-type, transient voltage-gated potassium channels. All three toxins have been shown to block the potassium channel Kv4.2.[2] Recombinant heteropodatoxin-2 blocks the potassium channels Kv4.1, Kv4.2 and Kv4.3, but not Kv1.4, Kv2.1, or Kv3.4.[3]
Mode of action
Heterpodatoxin-2 most likely acts as a gating modifier of the Kv4.2 channels.[3] It shifts the voltage dependence of the activation and the inactivation of the Kv4.3 potassium channel to more positive values. As a result, in the presence of the toxin this channel has a higher probability of being inactivated and a larger depolarization is needed to open the channel. However, heterpodatoxin-2 did not affect the voltage dependence of the Kv4.1 channel, suggesting that the precise mechanism of block remains to be elucidated,[3] and a role as a pore blocker cannot be excluded.[1] The voltage dependence of Kv4.2 block varies among the three different heteropodatoxins. It is less voltage dependent for HpTx1 than for HpTx2 or HpTx3.[2]
Toxicity
The giant crab spider can cause a locally painful bite.[4]
References
Bibliography
- "Solution structure of hpTX2, a toxin from Heteropoda venatoria spider that blocks Kv4.2 potassium channel". Protein Sci. 9 (11): 2059–67. 2000. PMID 11152117.
- "Heteropodatoxins: peptides isolated from spider venom that block Kv4.2 potassium channels". Mol. Pharmacol. 51 (3): 491–8. 1997. PMID 9058605.
- "Heteropoda toxin 2 is a gating modifier toxin specific for voltage-gated K+ channels of the Kv4 family". Toxicon 45 (4): 431–42. 2005. doi:10.1016/j.toxicon.2004.11.015. PMID 15733564.
 |