Chemistry:ImKTx88
ImKTx88 is a selective inhibitor of the Kv1 ion channel family that can be isolated from the venom of the Isometrus maculatus (lesser brown scorpion). This peptide belongs to the α-KTx subfamily and is classified as a pore-blocking toxin.
Etymology
The name ImKTx88 is derived from the scorpion species, Isometrus maculatus, its target, K channels, the abbreviation of toxin, Tx, and the clone number in the cDNA library, 88.[1][2]
Sources
ImKTx88 is a peptide that can be isolated from the venom produced by the glands of Isometrus maculatus (lesser brown scorpion).
Chemistry
Family and homology
ImKTx88 belongs to the α-KTx family.[1] This peptide has not shown a high percentage of sequence similarity with other toxins selective for the same family of K+ channels α-KTx and may be classified in a new group among the related Kv blocker.[1]
Structure
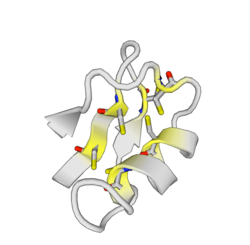
ImKTx88 complete sequence has a mass of 6,763 kDa and is constituted by 60 amino acids. The mature toxin, which misses the signal peptide of 22 amino acids, has a mass of 4,33 kDa and is constituted by 38 amino acids residues.[1][2] The peptide folds in three secondary structures: one α-helix at N-terminus, three anti-parallel β-sheets at C-terminus. The α-helix contains acidic residues, whereas β-sheets contain basic ones. Additionally, three disulphide bridges form the tertiary structure.[1] These structures patterns suggest that ImKTx88 may be a potential Kv channel blocker for its similarity with Kv channel blocker family. Although, its amino acid sequence is not very similar, the predicted folded protein was reasonably similar to most Kv channel blockers.[1] The typical aromatic ring with a lysine, found for alpha-KTX-family is present.[1]
Amino acid sequence:
MSNFSVFLIA LLFCSVFILS EAQIYTSKEC NGSSECYSHC EGITGKRSGK CINKKCYCYR
Target
ImKTx88 binds at the pore of the potassium channels Kv1.1, Kv1.2 and Kv1.3.[1] The basic residues on the anti-parallel β-sheets are implicated in this interaction. The toxin has a higher affinity for Kv1.3 than for Kv1.1 and Kv1.2, with an IC50 of 91 pM, 389 nM and 8,47 μM respectively.[1]
Mode of action
ImKTx88 is classified as a pore-blocking toxin.[1] ImKTx88 blocks Kv1.3 channels, which are present in dendritic cells,[3] lymphocytes,[4] mitral cells [5] and are also localized in the nucleus of several types of cells in cancer and in brain tissue.[6] Kv1.3 channels regulate the resting membrane potential, and the toxin might thus disrupt membrane repolarization and calcium signalling.[7]
Toxicity
Isometrus maculatus has been classified as mildly venomous species. Its sting displays a variety of neurotoxic symptoms like numbness and paraesthesia. The neurotoxic features may be related to the modulation of ion channels by the toxin.[8]
Therapeutic use
ImKTx88 ameliorate pathological severity in experimental autoimmune encephalomyelitis (EAE) rats by reducing extravasation into central nervous system (CNS) and by stabilizing the blood-brain barrier (BBB).[9] Moreover, this toxin improves the severity of loss or redistribution of tight junction proteins, and inhibits over-expression of ICAM-1 and VCAM-1 in the brain from EAE rats. ImKTx88 is being investigated as a novel therapeutic agent for multiple sclerosis (MS) treatment.[9] In macrophages, knock-down experiments of kcna3 (gene of Kv1.3) impaired cell growth and migration, features characteristic of cancer development [10]
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 1.7 1.8 1.9 Han, Song; Hu, Youtian; Zhang, Ruhong; Yi, Hong; Wei, Jingjing; Wu, Yingliang; Cao, Zhijian; Li, Wenxin et al. (February 2011). "ImKTx88, a novel selective Kv1.3 channel blocker derived from the scorpion Isometrus maculates". Toxicon 57 (2): 348–355. doi:10.1016/j.toxicon.2010.12.015. ISSN 1879-3150. PMID 21194541.
- ↑ 2.0 2.1 "Potassium channel toxin ImKTx88 precursor - Isometrus maculatus (Lesser brown scorpion)" (in en). https://www.uniprot.org/uniprot/P0CJ24.
- ↑ Mullen, Katherine M.; Rozycka, Monika; Rus, Horea; Hu, Lina; Cudrici, Cornelia; Zafranskaia, Ekaterina; Pennington, Michael W.; Johns, David C. et al. (July 2006). "Potassium channels Kv1.3 and Kv1.5 are expressed on blood-derived dendritic cells in the central nervous system". Annals of Neurology 60 (1): 118–127. doi:10.1002/ana.20884. ISSN 0364-5134. PMID 16729292.
- ↑ Szabò, Ildikò; Bock, Jurgen; Jekle, Andreas; Soddemann, Matthias; Adams, Constantin; Lang, Florian; Zoratti, Mario; Gulbins, Erich (2005-04-01). "A novel potassium channel in lymphocyte mitochondria". The Journal of Biological Chemistry 280 (13): 12790–12798. doi:10.1074/jbc.M413548200. ISSN 0021-9258. PMID 15632141.
- ↑ Tucker, Kristal; Cho, Sukhee; Thiebaud, Nicolas; Henderson, Michael X.; Fadool, Debra Ann (2013-05-15). "Glucose sensitivity of mouse olfactory bulb neurons is conveyed by a voltage-gated potassium channel". The Journal of Physiology 591 (10): 2541–2561. doi:10.1113/jphysiol.2013.254086. ISSN 1469-7793. PMID 23478133.
- ↑ Jang, Soo Hwa; Byun, Jun Kyu; Jeon, Won-Il; Choi, Seon Young; Park, Jin; Lee, Bo Hyung; Yang, Ji Eun; Park, Jin Bong et al. (2015-05-15). "Nuclear localization and functional characteristics of voltage-gated potassium channel Kv1.3". The Journal of Biological Chemistry 290 (20): 12547–12557. doi:10.1074/jbc.M114.561324. ISSN 1083-351X. PMID 25829491.
- ↑ Beeton, Christine; Wulff, Heike; Standifer, Nathan E.; Azam, Philippe; Mullen, Katherine M.; Pennington, Michael W.; Kolski-Andreaco, Aaron; Wei, Eric et al. (2006-11-14). "Kv1.3 channels are a therapeutic target for T cell-mediated autoimmune diseases". Proceedings of the National Academy of Sciences of the United States of America 103 (46): 17414–17419. doi:10.1073/pnas.0605136103. ISSN 0027-8424. PMID 17088564. Bibcode: 2006PNAS..10317414B.
- ↑ Quintero-Hernández, V.; Jiménez-Vargas, J. M.; Gurrola, G. B.; Valdivia, H. H.; Possani, L. D. (2013-12-15). "Scorpion venom components that affect ion-channels function". Toxicon 76: 328–342. doi:10.1016/j.toxicon.2013.07.012. ISSN 1879-3150. PMID 23891887.
- ↑ 9.0 9.1 Huang, Jie; Han, Song; Sun, Qi; Zhao, Yipeng; Liu, Junchen; Yuan, Xiaolu; Mao, Wenqian; Peng, Biwen et al. (2017). "Kv1.3 channel blocker (ImKTx88) maintains blood-brain barrier in experimental autoimmune encephalomyelitis". Cell & Bioscience 7: 31. doi:10.1186/s13578-017-0158-2. ISSN 2045-3701. PMID 28596825.
- ↑ Comes, Núria; Bielanska, Joanna; Vallejo-Gracia, Albert; Serrano-Albarrás, Antonio; Marruecos, Laura; Gómez, Diana; Soler, Concepció; Condom, Enric et al. (2013-10-10). "The voltage-dependent K(+) channels Kv1.3 and Kv1.5 in human cancer". Frontiers in Physiology 4: 283. doi:10.3389/fphys.2013.00283. ISSN 1664-042X. PMID 24133455.
 |