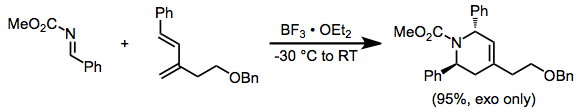Chemistry:Imine Diels–Alder reaction
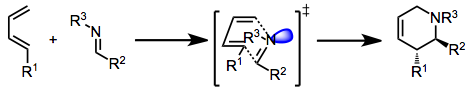
The imine Diels–Alder reaction involves the transformation of all-carbon dienes and imine dienophiles into tetrahydropyridines.[1]
Mechanism
The imino Diels-Alder (IDA) reaction may occur either by a concerted or stepwise process. The lowest-energy transition state for the concerted process places the imine lone pair (or coordinated Lewis acid) in an exo position. Thus, (E) imines, in which the lone pair and larger imine carbon substituent are cis, tend to give exo products.[2]
(2)
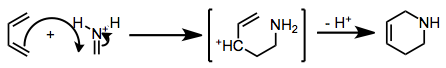
When the imine nitrogen is protonated or coordinated to a strong Lewis acid, the mechanism shifts to a stepwise, Mannich-Michael pathway.[3]
(3)
Stereoselective variants
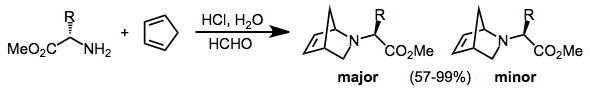
In many cases, cyclic dienes give higher diastereoselectivities than acyclic dienes. Use of amino-acid-based chiral auxiliaries, for instance, leads to good diastereoselectivities in reactions of cyclopentadiene, but not in reactions of acyclic dienes.[4]
(6)
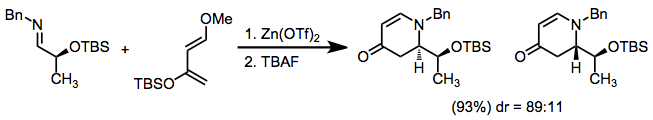
Chiral auxiliaries have been employed on either the imino nitrogen[5] or imino carbon[6] to effect diastereoselection.
(5)
Scope and limitations
Attaching an electron-withdrawing group to the imine nitrogen increases the reactivity of the imine. The exo isomer usually predominates (particularly when cyclic dienes are used), although selectivities vary.[7]
(7)
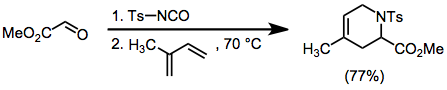
Tosylimines may be generated in situ from tosylisocyanate and aldehydes. Cycloadditions of these intermediates with dienes give single constitutional isomers, but proceed with moderate stereoselectivity.[8]
(8)
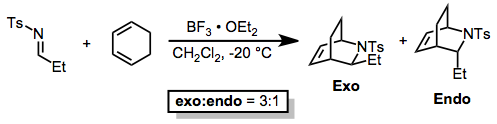
Lewis-acid catalyzed reactions of sulfonyl imines also exhibit moderate stereoselectivity.[9]
(9)
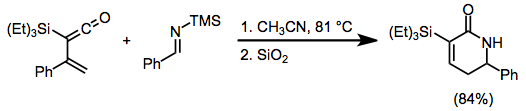
Simple unactivated imines react with hydrocarbon dienes only with the help of a Lewis acid; however, both electron-rich and electron-poor dienes react with unactivated imines when heated. Vinylketenes, for instance, afford dihydropyridones upon [4+2] cycloaddition with imines. Regio- and stereoselectivity are unusually high in reactions of this class of dienes.[10]
(10)
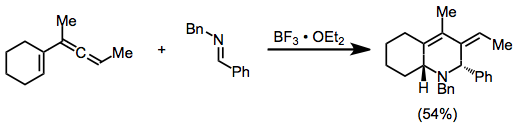
Vinylallenes react similarly in the presence of a Lewis acid, often with high diastereoselectivity.[11]
(11)
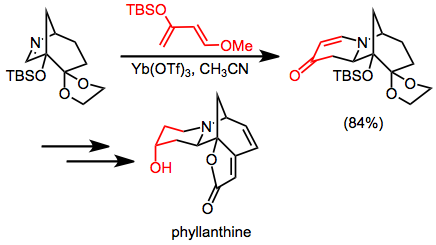
The IDA reaction has been applied to the synthesis of a number of alkaloid natural products. Danishefsky's diene is used to form a six-membered ring en route to phyllanthine.[12]
(12)
Actyliminium substrates
Acyliminium ions also participate in cycloadditions. These cations are generated by removal of chloride from chloromethylated amides:[13]
- RCONRCH
2Cl → RCONR/dCH+
2 + Cl−
The resulting acyl iminium cations serve as heterodienes as well as dienophile.
See also
- Oxo Diels–Alder reaction
- Aza-Diels–Alder reaction
References
- ↑ Heintzelman, G. R.; Meigh, I. R.; Mahajan, Y. R.; Weinreb, S. M. (2005). "Diels-Alder Reactions of Imino Dienophiles". Org. React. 65: 141–599. doi:10.1002/0471264180.or065.02. ISBN 0471264180.
- ↑ Whiting, A.; Windsor, C. M. (1998). "What makes a neutral imino dieneophile undergo a thermal, non-catalysed, Diels-Alder reaction?". Tetrahedron 54 (22): 6035. doi:10.1016/S0040-4020(98)00284-1.
- ↑ Hermitage, S.; Jay, D. A.; Whiting, A. (2002). "Evidence for the non-concerted \4+2]-cycloaddition of N-aryl imines when acting as both dienophiles and dienes under Lewis acid-catalysed conditions". Tetrahedron Lett. 43 (52): 9633. doi:10.1016/S0040-4039(02)02392-4.
- ↑ Waldmann, H. (1989). "Asymmetrische Hetero-Diels-Alder-Reaktionen in wäßriger Lösung unter Verwendung von Aminosäureestern als chiralen Auxiliaren". Liebigs Ann. Chem. 1989 (3): 231–238. doi:10.1002/jlac.198919890145.
- ↑ Hedberg, C.; Pinho, P.; Roth, P.; Andersson, P. G. (2000). "Diels-Alder reaction of heterocyclic imine dienophiles". J. Org. Chem. 65 (9): 2810–2. doi:10.1021/jo9916683. PMID 10808461.
- ↑ Ishimaru, K.; Watanabe, K.; Yamamoto, Y.; Akiba, K.-Y. (1994). "Stereocontrol in \4+2]Type Cycloaddition of an Aldimine Derived from (S)-Ethyl Lactate with 2-Siloxy-1,3-butadienes". Synlett 1994 (7): 495. doi:10.1055/s-1994-22902.
- ↑ Corey, E. J.; Yuen, P.-W. (1989). "A short, stereospecific route to chiral trans-2,6-disubstituted quinuclidines". Tetrahedron Lett. 30 (43): 5825. doi:10.1016/S0040-4039(01)93481-1.
- ↑ Schrader, T.; Steglich, W. (1990). "Phosphoranaloge von Aminosäuren IV.1Synthesen ungewöhnlicher 1-Aminophosphonsäuren über Diels-Alder-Reaktionen von (N-Acyliminomethyl)phosphonsäurediethylestern". Synthesis 1990 (12): 1153. doi:10.1055/s-1990-27122.
- ↑ Krow, G. R.; Pyun, C.; Rodebaugh, R.; Marakowski, J. (1974). "Heterodienophiles—V". Tetrahedron 30 (17): 2977. doi:10.1016/S0040-4020(01)97542-8.
- ↑ Bennett, D. M.; Okamoto, I.; Danheiser, R. L. (1999). "Hetero 4 + 2 cycloadditions of (trialkylsilyl)vinylketenes. Synthesis of alpha,beta-unsaturated delta-valerolactones and -lactams". Org. Lett. 1 (4): 641–4. doi:10.1021/ol9907217. PMID 10823193.
- ↑ Regas, D.; Afonso, M. M.; Rodriguez, M. L.; Palenzuela, J. A. (2003). "Synthesis of octahydroquinolines through the Lewis acid catalyzed reaction of vinyl allenes and imines". J. Org. Chem. 68 (20): 7845–52. doi:10.1021/jo034480z. PMID 14510565.
- ↑ Han, G.; LaPorte, M. G.; Folmer, J. J.; Werner, K. M.; Weinreb, S. M. (2000). "Total syntheses of the Securinega alkaloids (+)-14,15-dihydronorsecurinine, (−)-norsecurinine, and phyllanthine". J. Org. Chem. 65 (20): 6293–306. doi:10.1021/jo000260z. PMID 11052071.
- ↑ Weinreb, Steven M.; Scola, Paul M. (1989). "N-acyl imines and related hetero dienes in [4+2]-cycloaddition reactions". Chemical Reviews 89 (7): 1525–1534. doi:10.1021/cr00097a008.
 |