Chemistry:Isocytosine
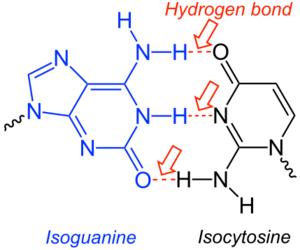
Isocytosine or 2-aminouracil is a pyrimidine base that is an isomer of cytosine. The nucleoside form is called isocytidine (iC).[1]
It is used in combination with isoguanine in studies of unnatural nucleic acid analogues of the normal base pairs in DNA.[2] In particular, it is used as a nucleobase of hachimoji RNA with the abbreviation rS.[3]
Synthesis
It can be synthesized from guanidine and malic acid.[4]
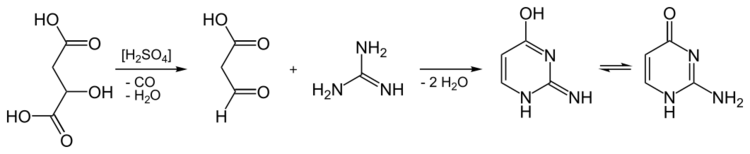
Isocytosine can also be obtained by condensing guanidine hydrochloride with 3-oxopropanoic acid. However, the C
3 component is not storable in this case and was, therefore, replaced with malic acid. This is decarbonylated in concentrated sulfuric acid with elimination of water, thus losing carbon monoxide. The 3-oxopropanoic acid formed in situ condenses with the guanidine in the sulfuric acid solution with double elimination of water.[5]
Uses
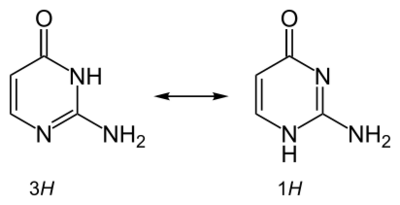
It is also used in physical chemical studies involving metal complex binding, hydrogen bonding, and tautomerism and proton transfer effects in nucleobases.[6]
References
- ↑ Hopfinger, MC; Kirkpatrick, CC; Znosko, BM (18 September 2020). "Predictions and analyses of RNA nearest neighbor parameters for modified nucleotides.". Nucleic Acids Research 48 (16): 8901–8913. doi:10.1093/nar/gkaa654. PMID 32810273.
- ↑ "Isocytosine". Molecule of the Week. American Chemical Society. http://portal.acs.org/portal/acs/corg/content?_nfpb=true&_pageLabel=PP_ARTICLEMAIN&node_id=841&content_id=CNBP_025023&use_sec=true&sec_url_var=region1&__uuid=46c9dd8e-b812-4a8d-9f18-18cb11f7a887. Retrieved November 1, 2012.
- ↑ Hoshika, Shuichi (22 February 2019). "Hachimoji DNA and RNA: A genetic system with eight building blocks". Science 363 (6429): 884–887. doi:10.1126/science.aat0971. PMID 30792304. Bibcode: 2019Sci...363..884H.
- ↑ William T. Caldwell , Harry B. Kime (1940). "A New Synthesis of Isocytosine". J. Am. Chem. Soc. 62 (9): 2365. doi:10.1021/ja01866a028. Bibcode: 1940JAChS..62.2365C.
- ↑ Caldwell, William T.; Kime, Harry B. (1 September 1940). "A New Synthesis of Isocytosine". Journal of the American Chemical Society 62 (9): 2365. doi:10.1021/ja01866a028. ISSN 0002-7863. Bibcode: 1940JAChS..62.2365C. https://pubs.acs.org/doi/abs/10.1021/ja01866a028. Retrieved 17 June 2025.
- ↑ "Isocytosine". Sigma-Aldrich. http://www.sigmaaldrich.com/catalog/product/sigma/i2127?lang=en. Retrieved November 1, 2012.
 |
