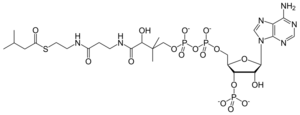
Chemistry:Isovaleryl-CoA
From HandWiki
| |
| Names | |
|---|---|
| IUPAC name
3′-O-Phosphonoadenosine 5′-[(3R)-3-hydroxy-2,2-dimethyl-4-{[3-({2-[(3-methylbutanoyl)sulfanyl]ethyl}amino)-3-oxopropyl]amino}-4-oxobutyl dihydrogen diphosphate]
| |
| Preferred IUPAC name
O1-{[(2R,3S,4R,5R)-5-(6-Amino-9H-purin-9-yl)-4-hydroxy-3-(phosphonooxy)oxolan-2-yl]methyl} O3-[(3R)-3-hydroxy-2,2-dimethyl-4-{[3-({2-[(3-methylbutanoyl)sulfanyl]ethyl}amino)-3-oxopropyl]amino}-4-oxobutyl] dihydrogen diphosphate | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| MeSH | isovaleryl-coenzyme+A |
PubChem CID
|
|
| |
| |
| Properties | |
| C26H44N7O17P3S | |
| Molar mass | 851.652 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
Isovaleryl-coenzyme A, also known as isovaleryl-CoA, is an intermediate in the metabolism of branched-chain amino acids.[1][2][3]
Leucine metabolism
See also
- Isovaleryl coenzyme A dehydrogenase
References
- ↑ 1.0 1.1 1.2 "International Society of Sports Nutrition Position Stand: beta-hydroxy-beta-methylbutyrate (HMB)". Journal of the International Society of Sports Nutrition 10 (1): 6. February 2013. doi:10.1186/1550-2783-10-6. PMID 23374455.
- ↑ 3.0 3.1 3.2 Nutrient Metabolism: Structures, Functions, and Genes (2nd ed.). Academic Press. May 2015. pp. 385–388. ISBN 978-0-12-387784-0. https://books.google.com/books?id=aTQTAAAAQBAJ&printsec=frontcover#v=onepage. Retrieved 6 June 2016. "Energy fuel: Eventually, most Leu is broken down, providing about 6.0kcal/g. About 60% of ingested Leu is oxidized within a few hours ... Ketogenesis: A significant proportion (40% of an ingested dose) is converted into acetyl-CoA and thereby contributes to the synthesis of ketones, steroids, fatty acids, and other compounds"
Figure 8.57: Metabolism of L-leucine
 |