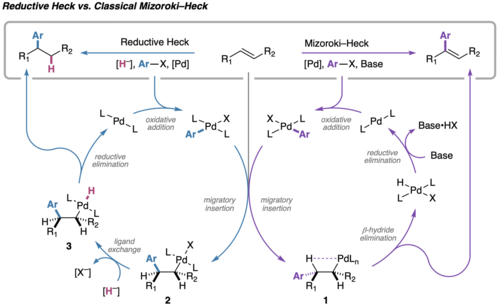
Chemistry:Mizoroki-Heck vs. Reductive Heck
The Mizoroki−Heck coupling of aryl halides and alkenes to form C(sp2)–C(sp2) bonds has become a staple transformation in organic synthesis, owing to its broad functional group compatibility and varied scope.[1][2][3][4][5][6][7][8][9][10] In stark contrast, the palladium-catalyzed reductive Heck reaction has received considerably less attention, despite the fact that early reports of this reaction date back almost half a century. From the perspective of retrosynthetic logic, this transformation is highly enabling because it can forge alkyl–aryl linkages from widely available alkenes, rather than from the less accessible and/or more expensive alkyl halide or organometallic C(sp3) synthons that are needed in a classical aryl/alkyl cross-coupling.
In part due to the historical difficulties of developing a generally applicable palladium(0)-catalyzed reductive Heck protocol that is compatible with diverse alkenes, various alternative strategies to achieve alkene hydroarylation have been developed. These include dual catalytic approaches,[11][12] reactions involving other metals,[13][14][15][16] and mechanistically distinct palladium-catalyzed methods.[17][18][19][20]
Mechanistic Overview
Based on various experimental observations, it has been proposed that the operative mechanism of the conventional Mizoroki−Heck reaction varies subtly depending on the reaction conditions;[21] however, the general mechanism is as follows: the catalytic cycle begins with oxidative addition of a C(sp2)–X (X = Br, Cl, I, OTf, etc.) bond to a palladium(0) complex followed by 1,2-migratory insertion to access an alkylpalladium(II) intermediate (1). This C(sp3)–PdII intermediate then succumbs to rapid β-hydride elimination to deliver the functionalized alkene product, followed by regeneration of palladium(0) via HX reductive elimination.
The reductive Heck reaction follows a similar mechanism, but involves intercepting the alkylpalladium(II) intermediate (2) with a hydride source (most commonly formate) to form a palladium complex (3) that can readily undergo reductive cleavage to form a new C–H bond.[22] Favoring the reductive pathway can be challenging due to competing β-hydride elimination; however, conformationally restricted olefins that lack β-hydrogens syn-periplanar relative to the C(sp3)–PdII can render β-hydride elimination inoperable. Additionally, substrates that have the ability to form stabilized π-allyl/π-benzyl/enolate intermediates can also react to give formal reductive Heck products. More recently, protocols have been developed that allow for reductive Heck coupling of unactivated aliphatic and heteroatom-substituted alkenes, which are discussed below.
Reductive Heck Reactions
Reactions with Strained Alkenes
In a seminal study in 1980, Catellani took advantage of the diastereospecificity of migratory insertion and β-hydride elimination with norbornene substrates, allowing interception of the alkylpalladium(II) intermediate 11.[23] In these systems, ligand exchange of the halide with formate results in formation of the norbornyl palladium species (12), which undergoes decarboxylation (12 → 13) and reductive elimination to afford the corresponding product. Later work improved the efficiency of the reaction with piperidine and tetraalkylammonium salt additives.[24]
Asymmetric reductive Heck couplings of norbornene scaffolds were first reported in 1991 using (R,R)-NorPhos, albeit with moderate enantioselectivity.[25] Subsequent reports found that enantioinduction could be improved through judicious choice of ligand (switching to P,N- or N,N- type ligands) and coupling partners (use of triflates over iodides).[26][27][28][29][30][31][32] The latter observation has been posited to arise from a suppression of competing reduction of the C(sp2)–PdII intermediate prior to alkene insertion.[33]
Following the successful development of asymmetric reductive Heck couplings on norbornene, this strategy was soon extended to oxanorbornene and azanorbornene substrates. Although the exact conditions and absolute configurations are not reported, Fiaud and coworkers reported an interesting observation regarding the reductive Heck arylation of 17.[34] Enantioselectivity was strongly influenced by the nature of the halide/pseudohalide employed, with aryl triflates coupling partners giving moderate enantiomeric excess (ee) while aryl iodides demonstrated no enantioinduction. This supports hypotheses of both cationic and neutral pathways analogous to the classic Mizoroki–Heck reaction [7, 8].[7][8] The asymmetric coupling of azanorbornene scaffolds has also been successful. In particular, the reductive Heck coupling of azanorbornene 19 has allowed for short, enantioselective syntheses of the alkaloid epibatidine and structural analogues [35–38].[35][36][37][38] Further, a strategy to access the natural product ibogaine and analogues (5)[39] involves an intramolecular reductive Heck reaction facilitated by a tether, a strategy that has also been extended to non-strained alkenyl systems.

Reactions with Styrenes
Reductive Heck hydroarylation of styrenes was first reported by Torii and colleagues in 1985.[40] The reaction gives hydroarylated products in good yields and with high regioselectivity, albeit only with conjugated olefins. Despite being one the first reports of a reductive Heck hydroarylation, Torii’s work remains the only example proceeding via an electro-reductive mechanism.
Recently, Jin, Hu, and coworkers reported a reductive Heck coupling between styrenyl substrates and aryl bromides (the conditions also allow for unactivated C(sp2)–C(sp3) coupling).[41] As shown by 21 and 22, methyl substitution was tolerated at the branched and terminal position of the alkene; however, phenyl substitution at these positions resulted in a significant decrease in yield, (19% and 10% for the branched and terminal positions, respectively). Other styrenyl-type substrates like 23 were compatible, as were some additional aryl bromide coupling partners (24). Kinetic and deuterium labeling experiments suggested that i-PrOH serves as the hydride source through β-H elimination.
Sigman and coworkers have developed a reductive Heck protocol for 1,3-dienes.[42] Based on previous work on similar systems, the authors posit that the transformation involves oxidative addition of an enol triflate or nonaflate with Pd(0) to form a cationic palladium complex that can undergo migratory insertion into a 1,3-diene. The migratory insertion intermediate (26) is in equilibrium with a π-allyl intermediate (25), which is subsequently trapped by the hydride source. Reductive elimination yields tri- and tetrasubstituted alkenes in moderate to good yields and selectivity.
Reactions with Tethered Alkenes
The bulk of the work completed on the reductive Heck reaction has been focused on enabling access to highly functionalized heterocyclic cores through a potentially enantioselective, transition-metal-catalyzed process. Larock’s seminal work on the preparation of indoline through a reductive Heck cyclization process[43] laid the groundwork for rapidly generating heterocycles. Although Larock did not employ a chiral ligand, recent advances in the field suggest that an asymmetric variant could be developed to afford enantioenriched indolines, structures that are of interest to the pharmaceutical industry [44, 45].[44][45]
The first asymmetric reductive Heck coupling of a tethered alkene was reported in 1998 by Diaz and coworkers en route to conformationally restricted retinoids.[46] This work only featured two examples and required the addition of calcium carbonate and silver-exchanged zeolites to give ee’s of 69% and 81%. Recent work by Zhang and coworkers using chiral sulfonamide phosphine ligands has improved the reaction to feature a broad substrate scope and high enantioselectivity (88–95% ee) without the use of stoichiometric silver additives.[47]
In 2007, Buchwald and coworkers reported the synthesis of 3-arylindanones via an asymmetric reductive Heck cyclization using chiral biaryl phosphine ligands to couple aryl triflates or nonaflates.[48] While pseudohalide substrates gave generally good yields and moderate enantioselectivity (50–94% ee), the use of aryl halides resulted in low conversion. The authors propose that hydride transfer to palladium occurs from the a-proton of the trialkylamine base, in this case Proton Sponge (1,8-bis(dimethylamino)naphthalene).
Later work by Zhou (54–97% ee with biaryl phosphine ligands)[49] and Minnaard (86–90% ee with monodentate phosphoramidite ligands)[50] extended the scope to aryl bromides and iodides, respectively; however, the aryl bromide substrates required 1 equivalent of benzoic acid additive in order to obtain high yields and enantioselectivity.
In their work on asymmetric reductive Heck cyclization to access 3,3-disubstituted oxindoles, Zhu and coworkers reported the only reductive Heck system to date that uses diboron/water as a hydride source.[51] Using a chiral phosphinooxazoline ligand to couple aryl triflates afforded the desired oxindole products in high yields and enantioselectivity (70–94% ee). Notably, deuterium-labeled compounds are accessible by using D2O in the reaction, allowing easy access to deuterated chiral oxindoles.
In 2015, Jia and colleagues reported an asymmetric dearomatization of indoles via an intramolecular reductive Heck reaction to yield quaternary indolines.[52] Using a chiral biaryl phosphine ligand and sodium formate (without trialkylamine additive) to couple aryl bromide substrates with a tethered indole moiety yielded the desired indoline products in moderate yields and high enantioselectivity (89–99% ee) in the absence of ortho-substitution on the bromobenzoyl ring. The presence of an ortho-methyl group resulted in significantly diminished yield (22%) and ee (29%). Subsequent work employing TMEDA/HCO2H as the reductant extended the scope of the reaction to tethered alkenyl bromides (93–99% ee).[53]
Recently, Tong and coworkers reported an asymmetric reductive Heck cyclization to afford quaternary tetrahydropyridines.[54] Using a chiral phosphinooxazoline ligand with DIPEA/HCO2H as the reductant, (Z)-1-iodo-1,6-dienes were cyclized to the corresponding tetrahydropyridines in good yields and enantioselectivity (71–99% ee). Notably, only 1,1-disubstituted and 1,1,2-trisubstituted alkenes afforded good yields and enantioselectivity. In addition, oxygen linked substrates exhibited similar reactivity, albeit with significantly diminished enantioselectivity (8–63% ee).
In 2019, Yao and colleagues reported a reductive Heck desymmetrization of cyclopentenes to access enantioenriched bicyclo[3.2.1]octanes.[55] A chiral bisphosphine ligand with sodium formate as the reductant yielded the desired products in good yields and high enantioselectivity. Like other systems, the presence of an ortho-methyl group (relative to the halogen) resulted in significantly diminished yield; however, the reaction was tolerant of a wide variety of other functional groups at various positions.

Reactions with Tethered Alkenes in Synthesis
The intramolecular reductive Heck cyclization of tethered alkenes has seen extensive use in total synthesis [56–63].[56][57][58][59][60][61][62][63] One such illustrative example is seen in the synthesis of ambiguine H and hapalindole U.[64][65] Baran and coworkers observed preferential formation of the undesired 7-endo-trig product and debromination when employing radical conditions on substrate 4; however, the desired 6-exo-trig cyclization was successfully observed when employing reductive Heck conditions. After extensive optimization, catalyst turnover remained relatively poor with various common palladium pre-catalysts, which the authors attributed to catalyst decomposition in the highly reducing environment. Slow addition of the more robust Herrmann’s palladacycle was found to elicit full consumption of the starting material to give product 5 in 65% isolated yield on gram-scale. A recent report by Snyder and coworkers uses almost identical reductive Heck conditions to construct a quaternary center en route to the conidiogenone natural products.[56]
In the Carreira synthesis of (±)-gelsemoxonine, a diastereoselective reductive Heck cyclization was used to form a key oxindole ring in 72% yield as a single diastereomer.[66] Notably, the reductive Heck conditions avoid undesired side reactivity including β-hydride elimination, destruction of the adjacent azetidine ring, and cleavage of the N−O and oxabicyclic C−O bonds.
Reactions with a,β-Unsaturated Enones/Enals
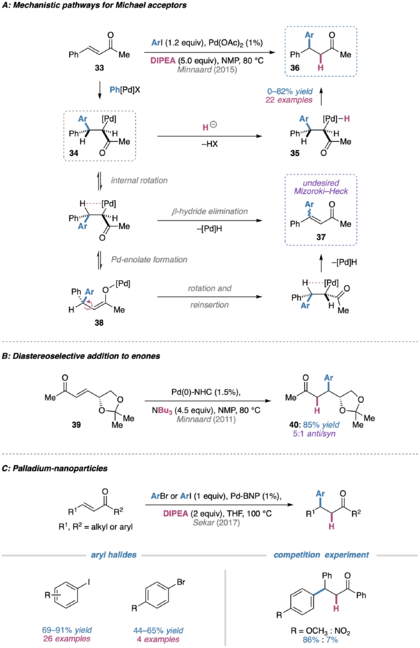
In 1983, Cacchi and coworkers disclosed a reductive Heck arylation of enones and enals in the presence of a trialkylamine base, tetrabutylammonium halide, and formic acid additive.[67] Under these conditions, the conjugate addition product is formed preferentially to the vinylic substitution (Mizoroki–Heck) product in high yield and selectivity. Notably, reductive Heck coupling on enones/enals features some mechanistically distinct aspects, as described in studies by Cacchi and later Minnaard.[68][69][70][71][72][73][74][75]
Both the conjugate addition and vinylic substitution mechanisms proceed through a common alkylpalladium(II) intermediate 34. In the case of vinylic substitution, internal bond rotation can result in the required syn-periplanar geometry necessary for β-hydride elimination to deliver the functionalized alkene product 37. A mixture of E/Z isomers is obtained due to the formation of a palladium enolate species (38), which facilitates reinsertion without facial preference.
In the conjugate addition case, intermediate 34 can be intercepted with formic acid to form a palladium complex (35) that can readily undergo reductive cleavage to form the new C–H bond. Control experiments run with added Heck product have ruled out the occurrence of a tandem Mizoroki–Heck reaction followed by reduction of the alkene by a palladium–hydride species; furthermore, computational studies suggest that reductive cleavage of Pd (rather than protonolysis) results in the formation of the product.
Building on Cacchi’s original conditions, Minnaard and coworkers have introduced systems that do not require the addition of formic acid or tributylamine additives.[76] In Pd(OAc)2 or Pd(TFA)2 catalyzed reductive Heck reactions with aryl iodide coupling partners, N,N-diisopropylethylamine (DIPEA) can serve as the reductant.[77][78][79][80][81] When using NMP as the solvent, electron-rich and electron-neutral aryl iodides gave good selectivity and moderate yields when coupling to enones with aryl/bulky substituents; however, selectivity and yield was diminished when coupling electron-deficient aryl iodides and when substrates featured non-bulky alkyl substituents on the β-carbon.
In a related system, N-heterocyclic carbene palladium complexes can be used in combination with various reductants in NMP or DMF to generate conjugate addition products.[82] The nature of the base has been shown to govern the course of the reaction, allowing preferential formation of either the classical Mizoroki–Heck or reductive Heck products. When applied to D-mannitol-derived substrate 39, the desired product 40 was formed diastereoselectively (5:1 anti/syn). Unfortunately, attempts to render the reductive Heck reaction enantioselective with both the N-heterocyclic carbene palladium complex and palladium acetate systems were not fruitful.
Recently, reductive Heck conjugate additions catalyzed by binaphthyl-backbone-stabilized palladium nanoparticles have been reported.[83] The reusable palladium nanocatalyst afforded good yields for both electron-rich and electron-poor aryl iodides as well as moderate yields for selected aryl bromides. While good yields are possible with both electron-rich and electron-poor aryl iodides, competition experiments showed that the rate is significantly faster for electron-rich aryl iodides.
Reactions with Terminal and Unactivated Alkenes
While early reports of an intermolecular reductive Heck required activated alkenes or systems that lacked β-hydrogens, the recent development of a process employing terminal alkenes and iodoarenes has altered the landscape and offered new opportunities for advancing this promising methodology.
Chemists at Merck identified an opportunity to use a reductive Heck reaction in their retrosynthetic analysis of NK-1 receptor antagonist precursors.[84] The authors hypothesized that steric considerations in dihydrofuran 41 would limit the ability of the alkylpalladium intermediate to undergo β-hydride elimination (similar to the strategy invoked in the earlier discussed strained alkenes). This strategy did indeed furnish the desired product with the correct regio- and stereochemistry; however, the authors note that excess lithium chloride additive was required to prevent competing dehalogenation of the aryl iodide.[85] Notably, this is one of few examples of a diastereoselective intermolecular reductive Heck.
In 2018, Engle and coworkers disclosed an intermolecular reductive Heck reaction of diverse terminal alkenes and select internal alkenes utilizing aqueous tetramethylammonium formate as the reductant.[86] This method tolerated an array of functional groups, including reductively labile groups, on both the (hetero)aryl iodide and alkene coupling partners, and was generally regioselective for the anti-Markovnikov product for terminal alkenes. A variety of terminal alkenes were compatible with the conditions, including simple a-olefins, heteroatom-substituted alkenes, and alkene-containing complex molecules such as quinine (49) and various terpenes.
In addition, cyclic internal alkenes (45 and 48) were suitable substrates, affording reasonable yields of product; however acyclic internal alkenes were poor substrates for the reaction in terms of yield and regioselectivity. Unique to this method, the authors discovered that a 10:1 phosphine to palladium loading was essential to achieve high yields and suppress the formation of Heck byproducts. The authors hypothesized that the high phosphine loading leads to coordinative saturation of the palladium center to prevent β-hydride elimination from the alkylpalladium(II) intermediate, allowing it to decarboxylate formate to produce a palladium–hydride that could reductively eliminate to give the desired product.
Recently, Wu, Loh, and coworkers reported an auxiliary-directed reductive Heck reaction of unactivated alkenes and aryl triflates utilizing proton sponge as the hydride source.[87] The authors rely on an 8-aminoquinoline directing group to control the regioselectivity of migratory insertion and stabilize the resulting alkylpalladium(II) intermediate (50). Both terminal β,γ- and γ,δ-alkenyl carbonyl compounds were suitable substrates for the reaction; however, internal alkenes proved to be more challenging to functionalize, with only β,γ-internal alkenes yielding products.
As previously mentioned, Jin, Hu, and coworkers developed a reductive Heck reaction of aryl bromides with styrenes and unactivated alkenes.[41] The reaction requires a preformed palladium catalyst consisting of a specialized constrained bidentate iminopyridyl (CImPy) ligand, which is believed to be vital for stabilizing the palladium center. The authors found that several simple α-olefins were suitable substrates for the reaction, providing reasonable yields with moderate regioselectivities. Both symmetric linear and cyclic alkenes were compatible substrates, although increasing the ring size of the cycloalkene resulted in diminished yields (55 vs. 56). In addition, using 2,3-dihydrofuran as a substrate afforded the 2-substituted tetrahydrofuran product (52) in good yield and high regioselectivity.

Prospects for Applications in the Pharmaceutical Industry
As is evident from the examples above, reductive Heck hydroarylation can be an enabling disconnection that affords structures similar to those derived from other sp2-sp3 cross-coupling reactions, but with the benefit of simplifying the required starting material (in this case an alkene synthon). This synthetic logic holds substantial promise for applications in the pharmaceutical industry, despite its relatively limited application to date.
New synthetic methodologies are constantly required in the pharmaceutical sector due to the increasing diversity of chemical modalities used to treat human disease. The rate of exploration of new chemical space has significantly increased over the last few decades, which increases the complexity of the chemical structures being pursued.[88] This continuing evolution represents a consistent challenge to pharmaceutical chemists and demands new bond forming processes. The structural complexity of a molecule is often linked to the complexity of its synthesis, features which can be combined and measured by determining the molecule’s current complexity.[89] In this context, developing commercially viable, efficient and sustainable synthetic routes to these compounds requires both innovation in strategy and capability. To address these challenges, chemists in the pharmaceutical sector have applied the concept of disruptive innovation,[90] innovation that delivers a step change in the efficiency of preparing a molecule, in their approach to route development. Thus, the discovery, development, and rapid application of new synthetic methodologies, such as the reductive Heck reaction, can significantly enhance chemists’ capability to prepare novel drug candidates.
As previously mentioned, Larock’s seminal work laid the groundwork for the application of the reductive Heck to rapid generation of heterocycles.[43] In the years following Larock’s report, the asymmetric synthesis of dihydrobenzofurans,[47] indanones,[48][49][50] oxindoles,[51] quaternary indolines,[52][53] and tetrahydropyridines,[54] has been reported. All of these cores have been important substructures in the development of new drug candidates. 3-Arylindanones were reported to have anticancer activity,[91] and as of 2017, six different oxindole core structures were in clinical trials for over fifteen different indications.[92] Hence, the development of new methods for applying reductive-Heck-like processes to these heterocyclic systems could have a significant impact on the development of commercially viable routes to many different clinical candidates.
While the intermolecular reductive Heck reaction has seen some limited usage in the pharmaceutical industry (synthesis of NK-1 receptor antagonist precursors)[84] and drug discovery,[93][94][95][96][97][98][99][100] the continued development of reductive Heck reactions on terminal and unactivated alkenes represents a valuable new disconnection in medicinal chemistry. The formation of an C(sp2)–C(sp3) bond between simple aromatic halides and terminal alkenes affords functionalized intermediates from simple, commercially available, inexpensive reagents,[86] and has the potential to replace Negishi and Suzuki couplings as the preferred disconnection for these advanced intermediates. Further, the reductive Heck reaction of unactivated alkenes with aryl bromides has been reported in good yield and moderate to good selectivity,[41] suggesting that the vast array of aryl bromides will soon be suitable partners in a reductive Heck transformation.
References
- ↑ Fujiwara, Yuzo; Moritani, Ichiro; Danno, Sadao; Asano, Ryuzo; Teranishi, Shiichiro (December 1969). "Aromatic substitution of olefins. VI. Arylation of olefins with palladium(II) acetate". Journal of the American Chemical Society 91 (25): 7166–7169. doi:10.1021/ja01053a047. ISSN 0002-7863. PMID 27462934.
- ↑ Mizoroki, Tsutomu; Mori, Kunio; Ozaki, Atsumu (February 1971). "Arylation of Olefin with Aryl Iodide Catalyzed by Palladium". Bulletin of the Chemical Society of Japan 44 (2): 581. doi:10.1246/bcsj.44.581. ISSN 0009-2673.
- ↑ Heck, R. F.; Nolley, J. P. (July 1972). "Palladium-catalyzed vinylic hydrogen substitution reactions with aryl, benzyl, and styryl halides". The Journal of Organic Chemistry 37 (14): 2320–2322. doi:10.1021/jo00979a024. ISSN 0022-3263.
- ↑ Heck, Richard F. (1979-04-01). "Palladium-catalyzed reactions of organic halides with olefins". Accounts of Chemical Research 12 (4): 146–151. doi:10.1021/ar50136a006. ISSN 0001-4842.
- ↑ Cabri, Walter; Candiani, Ilaria (1995-01-01). "Recent Developments and New Perspectives in the Heck Reaction". Accounts of Chemical Research 28 (1): 2–7. doi:10.1021/ar00049a001. ISSN 0001-4842.
- ↑ Beletskaya, Irina P.; Cheprakov, Andrei V. (2000-08-01). "The Heck Reaction as a Sharpening Stone of Palladium Catalysis". Chemical Reviews 100 (8): 3009–3066. doi:10.1021/cr9903048. ISSN 0009-2665. PMID 11749313.
- ↑ 7.0 7.1 Dounay, Amy B.; Overman, Larry E. (2009), "The Asymmetric Intramolecular Mizoroki–Heck Reaction in Natural Product Total Synthesis", The Mizoroki–Heck Reaction, John Wiley & Sons, Ltd, pp. 533–568, doi:10.1002/9780470716076.ch16, ISBN 9780470716076
- ↑ 8.0 8.1 Mc Cartney, Dennis; Guiry, Patrick J. (2011). "The asymmetric Heck and related reactions" (in en). Chemical Society Reviews 40 (10): 5122–50. doi:10.1039/c1cs15101k. ISSN 0306-0012. PMID 21677934.
- ↑ Johansson Seechurn, Carin C. C.; Kitching, Matthew O.; Colacot, Thomas J.; Snieckus, Victor (2012). "Palladium-Catalyzed Cross-Coupling: A Historical Contextual Perspective to the 2010 Nobel Prize" (in en). Angewandte Chemie International Edition 51 (21): 5062–5085. doi:10.1002/anie.201107017. ISSN 1521-3773. PMID 22573393.
- ↑ Rauf, Waqar; Brown, John M. (2013). "Reactive intermediates in catalytic alkenylation; pathways for Mizoroki–Heck, oxidative Heck and Fujiwara–Moritani reactions". Chemical Communications 49 (76): 8430–40. doi:10.1039/c3cc44842h. ISSN 1359-7345. PMID 23949625.
- ↑ Friis, Stig D.; Pirnot, Michael T.; Buchwald, Stephen L. (2016-07-13). "Asymmetric Hydroarylation of Vinylarenes Using a Synergistic Combination of CuH and Pd Catalysis". Journal of the American Chemical Society 138 (27): 8372–8375. doi:10.1021/jacs.6b04566. ISSN 0002-7863. PMID 27346525.
- ↑ Friis, Stig D.; Pirnot, Michael T.; Dupuis, Lauren N.; Buchwald, Stephen L. (2017-06-12). "A Dual Palladium and Copper Hydride Catalyzed Approach for Alkyl-Aryl Cross-Coupling of Aryl Halides and Olefins" (in en). Angewandte Chemie International Edition 56 (25): 7242–7246. doi:10.1002/anie.201703400. PMID 28510287.
- ↑ Coombs, John R.; Morken, James P. (2016-01-13). "Catalytic Enantioselective Functionalization of Unactivated Terminal Alkenes". Angewandte Chemie International Edition 55 (8): 2636–2649. doi:10.1002/anie.201507151. ISSN 1433-7851. PMID 26764019.
- ↑ Crossley, Steven W. M.; Obradors, Carla; Martinez, Ruben M.; Shenvi, Ryan A. (2016-07-27). "Mn-, Fe-, and Co-Catalyzed Radical Hydrofunctionalizations of Olefins". Chemical Reviews 116 (15): 8912–9000. doi:10.1021/acs.chemrev.6b00334. ISSN 0009-2665. PMID 27461578.
- ↑ Nguyen, Julia; Chong, Andrea; Lalic, Gojko (2019). "Nickel-catalyzed anti-Markovnikov hydroarylation of alkenes". Chemical Science 10 (11): 3231–3236. doi:10.1039/c8sc05445b. ISSN 2041-6520. PMID 30996906.
- ↑ Chen, Yue-Gang; Shuai, Bin; Xu, Xue-Tao; Li, Yi-Qian; Yang, Qi-Liang; Qiu, Hui; Zhang, Kun; Fang, Ping et al. (2019-02-27). "Nickel-catalyzed Enantioselective Hydroarylation and Hydroalkenylation of Styrenes". Journal of the American Chemical Society 141 (8): 3395–3399. doi:10.1021/jacs.8b13524. ISSN 0002-7863. PMID 30741543.
- ↑ McDonald, Richard I.; Liu, Guosheng; Stahl, Shannon S. (2011-03-23). "Palladium(II)-Catalyzed Alkene Functionalization via Nucleopalladation: Stereochemical Pathways and Enantioselective Catalytic Applications". Chemical Reviews 111 (4): 2981–3019. doi:10.1021/cr100371y. ISSN 0009-2665. PMID 21428440.
- ↑ Mei, Tian-Sheng; Werner, Erik W.; Burckle, Alexander J.; Sigman, Matthew S. (2013-04-24). "Enantioselective Redox-Relay Oxidative Heck Arylations of Acyclic Alkenyl Alcohols using Boronic Acids". Journal of the American Chemical Society 135 (18): 6830–6833. doi:10.1021/ja402916z. ISSN 0002-7863. PMID 23607624.
- ↑ Zhang, Chun; Santiago, Celine B.; Crawford, Jennifer M.; Sigman, Matthew S. (2015-12-23). "Enantioselective Dehydrogenative Heck Arylations of Trisubstituted Alkenes with Indoles to Construct Quaternary Stereocenters". Journal of the American Chemical Society 137 (50): 15668–15671. doi:10.1021/jacs.5b11335. ISSN 0002-7863. PMID 26624236.
- ↑ Mei, Tian-Sheng; Patel, Harshkumar H.; Sigman, Matthew S. (April 2014). "Enantioselective construction of remote quaternary stereocentres". Nature 508 (7496): 340–344. doi:10.1038/nature13231. ISSN 0028-0836. PMID 24717439. Bibcode: 2014Natur.508..340M.
- ↑ Kürti, László (2007). Strategic applications of named reactions in organic synthesis : background and detailed mechanisms ; 250 named reactions. Elsevier Academic Press. ISBN 9780124297852. OCLC 263451014.
- ↑ Cacchi, S. (2009). "The palladium-catalyzed hydroarylation and hydrovinylation of carbon-carbon multiple bonds: New perspectives in organic synthesis". Pure and Applied Chemistry 62 (4): 713–722. doi:10.1351/pac199062040713. ISSN 1365-3075.
- ↑ Catellani, Marta; Chiusoli, G. Paolo; Giroldini, William; Salerno, Giuseppe (1980-10-21). "New transition metal-catalyzed CC coupling reactions initiated by CX bond cleavage and terminated by H-transfer". Journal of Organometallic Chemistry 199 (1): C21–C23. doi:10.1016/S0022-328X(00)84537-4. ISSN 0022-328X.
- ↑ Arcadi, A.; Marinelli, F.; Bernocchi, E.; Cacchi, S.; Ortar, G. (1989-06-06). "Palladium-catalyzed preparation of exo-aryl derivatives of the norbornane skeleton". Journal of Organometallic Chemistry 368 (2): 249–256. doi:10.1016/0022-328X(89)85320-3. ISSN 0022-328X.
- ↑ Brunner, H.; Kramler, K. (1991). "Asymmetric Catalysis. 72. 1 Enantioselective Hydroarylation of Norbornene and Norbornadiene with Palladium(II) Acetate/Phosphine Catalysts" (in en). Synthesis 1991 (12): 1121–1124. doi:10.1055/s-1991-28402. ISSN 0039-7881.
- ↑ Sakuraba, Shunji; Awano, Katsuya; Achiwa, Kazuo (1994). "Asymmetric Heck-type Hydroarylation of Norbornene with Phenyl Triflate Catalyzed by Palladium Complexes of Chiral (β- N -Sulfonylaminoalkyl)phosphines 1" (in en). Synlett 1994 (4): 291–292. doi:10.1055/s-1994-22833. ISSN 0936-5214.
- ↑ Namyslo, Jan Christoph; Kaufmann, Dieter E. (September 1997). "Palladium — Catalyzed Reactions, 1 Palladium-Catalyzed Enantioselective Hydrophenylation and Hydrohetarylation of Bicyclo[2.2.1]Hept-2-ene: Influence of the Chiral Ligand, the Leaving Group, and the Solvent" (in de). Chemische Berichte 130 (9): 1327–1331. doi:10.1002/cber.19971300924.
- ↑ Wu, Xin-Yan; Xu, Hua-Dong; Zhou, Qi-Lin; Chan, Albert S.C. (April 2000). "Enantioselective Heck-type hydroarylation of norbornene with phenyl iodide catalyzed by palladium/quinolinyl-oxazolines". Tetrahedron: Asymmetry 11 (6): 1255–1257. doi:10.1016/s0957-4166(00)00067-7. ISSN 0957-4166.
- ↑ Dupont, Jairton; Ebeling, Gunter; Delgado, Marcelo R.; Consorti, Crestina S.; Burrow, Robert; Farrar, David H.; Lough, Alan J. (2001-09-01). "A palladium complex containing a new C2-symmetric bidentate non-racemic oxalamidine ligand: synthesis and catalytic properties". Inorganic Chemistry Communications 4 (9): 471–474. doi:10.1016/S1387-7003(01)00249-0. ISSN 1387-7003.
- ↑ Wu, Xin-Yan; Xu, Hua-Dong; Tang, Fang-Yi; Zhou, Qi-Lin (October 2001). "Asymmetric hydroarylation of norbornene derivatives catalyzed by palladium complexes of chiral quinolinyl-oxazolines". Tetrahedron: Asymmetry 12 (18): 2565–2569. doi:10.1016/s0957-4166(01)00436-0. ISSN 0957-4166.
- ↑ Drago, Daniela; Pregosin, Paul S. (March 2002). "Palladium−Duphos Structural and Enantioselective Hydroarylation Chemistry". Organometallics 21 (6): 1208–1215. doi:10.1021/om0108898. ISSN 0276-7333.
- ↑ Namyslo, Jan C.; Storsberg, Jörg; Klinge, Jens; Gärtner, Christian; Yao, Min-Liang; Ocal, Nuket; Kaufmann, Dieter Eckhard (2010-05-10). "The Hydroarylation Reaction—Scope and Limitations". Molecules 15 (5): 3402–3410. doi:10.3390/molecules15053402. ISSN 1420-3049. PMID 20657489.
- ↑ Ozawa, Fumiyuki; Kobatake, Yosuhiro; Kubo, Akihiko; Hayashi, Tamio (1994). "Palladium-catalysed asymmetric hydroalkenylation of norbornene" (in en). Journal of the Chemical Society, Chemical Communications (11): 1323–1324. doi:10.1039/c39940001323. ISSN 0022-4936. http://xlink.rsc.org/?DOI=c39940001323.
- ↑ Moinet, Christophe; Fiaud, Jean-Claude (March 1995). "Palladium-catalyzed asymmetric hydrophenylation of 1,4-dihydro-1,4-epoxynaphthalene". Tetrahedron Letters 36 (12): 2051–2052. doi:10.1016/0040-4039(95)00178-f. ISSN 0040-4039.
- ↑ Namyslo, Jan Christoph; Kaufmann, Dieter E. (June 1999). "Asymmetric Synthesis of Both Enantiomers of N-Protected Epibatidine via Reductive Heck-Type Hetarylation". Synlett 1999 (6): 804–806. doi:10.1055/s-1999-2719. ISSN 0936-5214.
- ↑ Li, Xiao-Guang; Tang, Fang-Yi; Xu, Hua-Dong; Wu, Xin-Yan; Zhou, Qi-Lin (2002-11-23). "Asymmetric hydroarylation of hetero-atom containing norbornene derivatives and enantioselective synthesis of analogs of epibatidine" (in en). Arkivoc 2003 (2): 15–20. doi:10.3998/ark.5550190.0004.203. ISSN 1551-7012.
- ↑ Kasyan, Andrey; Wagner, Christoph; Maier, Martin E. (July 1998). "Regiochemistry of the reductive Heck coupling of 2-azabicyclo[2.2.1]hept-5-ene. Synthesis of epibatidine analogues". Tetrahedron 54 (28): 8047–8054. doi:10.1016/s0040-4020(98)00443-8. ISSN 0040-4020.
- ↑ Cox, Caroline D.; Malpass, John R. (October 1999). "Synthesis of epibatidine isomers: Reductive Heck coupling of 2-azabicyclo[2.2.1]hept-5-ene derivatives". Tetrahedron 55 (40): 11879–11888. doi:10.1016/s0040-4020(99)00675-4. ISSN 0040-4020.
- ↑ Jana, Goutam Kumar; Sinha, Surajit (May 2012). "Corrigendum to "Reductive Heck coupling: an efficient approach toward the iboga alkaloids. Synthesis of ibogamine, epiibogamine, and iboga analogs" [Tetrahedron Lett. 53 (2012) 1671–1674]". Tetrahedron Letters 53 (20): 2575. doi:10.1016/j.tetlet.2012.03.029. ISSN 0040-4039.
- ↑ Torii, Sigeru; Tanaka, Hideo; Morisaki, Kazuo (1985-09-05). "Pd(0)-CATALYZED ELECTRO-REDUCTIVE HYDROCOUPLING OF ARYL HALIDES WITH OLEFINS AND ACETYLENES". Chemistry Letters 14 (9): 1353–1354. doi:10.1246/cl.1985.1353. ISSN 0366-7022.
- ↑ 41.0 41.1 41.2 Jin, Liqun; Qian, Jiaxia; Sun, Nan; Hu, Baoxiang; Shen, Zhenlu; Hu, Xinquan (2018). "Pd-Catalyzed reductive heck reaction of olefins with aryl bromides for Csp 2 –Csp 3 bond formation" (in en). Chemical Communications 54 (45): 5752–5755. doi:10.1039/C8CC02571A. ISSN 1359-7345. PMID 29781006.
- ↑ Saini, Vaneet; O’Dair, Mark; Sigman, Matthew S. (2015-01-21). "Synthesis of Highly Functionalized Tri- and Tetrasubstituted Alkenes via Pd-Catalyzed 1,2-Hydrovinylation of Terminal 1,3-Dienes". Journal of the American Chemical Society 137 (2): 608–611. doi:10.1021/ja511640g. ISSN 0002-7863. PMID 25555197.
- ↑ 43.0 43.1 Larock, Richard C.; Babu, Srinivasan (January 1987). "Synthesis of nitrogen heterocycles via palladium-catalyzed intramolecular cyclization". Tetrahedron Letters 28 (44): 5291–5294. doi:10.1016/s0040-4039(00)96710-8. ISSN 0040-4039.
- ↑ Abdel-Magid, Ahmed F. (2017-01-12). "Viral Replication Inhibitors May Treat the Dengue Virus Infections". ACS Medicinal Chemistry Letters 8 (1): 14–16. doi:10.1021/acsmedchemlett.6b00513. PMID 28105266.
- ↑ Patent US2018/0099933
- ↑ Diaz, Philippe; Gendre, Fabrice; Stella, Lucien; Charpentier, Bruno (April 1998). "New synthetic retinoids obtained by palladium-catalyzed tandem cyclisation-hydride capture process". Tetrahedron 54 (18): 4579–4590. doi:10.1016/s0040-4020(98)00169-0. ISSN 0040-4020.
- ↑ 47.0 47.1 Zhang, Zhan-Ming; Xu, Bing; Qian, Yanyan; Wu, Lizuo; Wu, Yuanqi; Zhou, Lujia; Liu, Yu; Zhang, Junliang (2018). "Palladium-Catalyzed Enantioselective Reductive Heck Reactions: Convenient Access to 3,3-Disubstituted 2,3-Dihydrobenzofuran" (in en). Angewandte Chemie International Edition 57 (32): 10373–10377. doi:10.1002/anie.201806372. ISSN 1521-3773. PMID 29923656.
- ↑ 48.0 48.1 Minatti, Ana; Zheng, Xiaolai; Buchwald, Stephen L. (November 2007). "Synthesis of Chiral 3-Substituted Indanones via an Enantioselective Reductive-Heck Reaction". The Journal of Organic Chemistry 72 (24): 9253–9258. doi:10.1021/jo701741y. ISSN 0022-3263. PMID 17967034.
- ↑ 49.0 49.1 Yue, Guizhou; Lei, Kaining; Hirao, Hajime; Zhou, Jianrong (Steve) (2015). "Palladium-Catalyzed Asymmetric Reductive Heck Reaction of Aryl Halides" (in en). Angewandte Chemie International Edition 54 (22): 6531–6535. doi:10.1002/anie.201501712. ISSN 1521-3773. PMID 25867113.
- ↑ 50.0 50.1 Mannathan, Subramaniyan; Raoufmoghaddam, Saeed; Reek, Joost N. H.; de Vries, Johannes G.; Minnaard, Adriaan J. (2017). "Enantioselective Intramolecular Reductive Heck Reaction with a Palladium/Monodentate Phosphoramidite Catalyst" (in en). ChemCatChem 9 (4): 551–554. doi:10.1002/cctc.201601153. ISSN 1867-3899. https://dare.uva.nl/personal/pure/en/publications/enantioselective-intramolecular-reductive-heck-reaction-with-a-palladiummonodentate-phosphoramidite-catalyst(1ce4d556-31d7-4a23-9c4e-e5dc4ce408f1).html.
- ↑ 51.0 51.1 Kong, Wangqing; Wang, Qian; Zhu, Jieping (2017). "Water as a Hydride Source in Palladium-Catalyzed Enantioselective Reductive Heck Reactions" (in en). Angewandte Chemie International Edition 56 (14): 3987–3991. doi:10.1002/anie.201700195. ISSN 1521-3773. PMID 28272769.
- ↑ 52.0 52.1 Shen, Chong; Liu, Ren-Rong; Fan, Ren-Jie; Li, Ying-Long; Xu, Teng-Fei; Gao, Jian-Rong; Jia, Yi-Xia (2015-04-22). "Enantioselective Arylative Dearomatization of Indoles via Pd-Catalyzed Intramolecular Reductive Heck Reactions". Journal of the American Chemical Society 137 (15): 4936–4939. doi:10.1021/jacs.5b01705. ISSN 0002-7863. PMID 25849154.
- ↑ 53.0 53.1 Liang, Ren-Xiao; Yang, Run-Ze; Liu, Ren-Rong; Jia, Yi-Xia (2018). "Palladium-catalyzed asymmetric dearomative alkenylation of indoles through a reductive-Heck reaction" (in en). Organic Chemistry Frontiers 5 (11): 1840–1843. doi:10.1039/C8QO00205C. ISSN 2052-4129.
- ↑ 54.0 54.1 Hou, Longlei; Yuan, Yuejie; Tong, Xiaofeng (2017). "Pd(0)-Catalysed asymmetric reductive Heck-type cyclization of (Z)-1-iodo-1,6-dienes and enantioselective synthesis of quaternary tetrahydropyridines" (in en). Organic & Biomolecular Chemistry 15 (22): 4803–4806. doi:10.1039/C7OB00762K. ISSN 1477-0520. PMID 28548157.
- ↑ Yuan, Zhenbo; Feng, Ziwen; Zeng, Yuye; Zhao, Xiaobin; Lin, Aijun; Yao, Hequan (2019). "Palladium-Catalyzed Asymmetric Intramolecular Reductive Heck Desymmetrization of Cyclopentenes: Access to Chiral Bicyclo[3.2.1]octanes" (in en). Angewandte Chemie International Edition 58 (9): 2884–2888. doi:10.1002/anie.201900059. ISSN 1521-3773. PMID 30664333.
- ↑ 56.0 56.1 Snyder, Scott A.; Hsu, Ian Tingyung; Keim, Jonathan H.; Gong, Xu; DeBacker, Kenneth C.; Chi, Hyung Min; Hu, Pengfei (2019-04-25). "Quaternary-centre-guided synthesis of complex polycyclic terpenes" (in en). Nature 569 (7758): 703–707. doi:10.1038/s41586-019-1179-2. ISSN 1476-4687. PMID 31022719. Bibcode: 2019Natur.569..703H.
- ↑ Trost, Barry M.; Toste, F. Dean (1999-04-01). "Palladium-Catalyzed Kinetic and Dynamic Kinetic Asymmetric Transformation of 5-Acyloxy-2-(5H)-furanone. Enantioselective Synthesis of (−)-Aflatoxin B Lactone". Journal of the American Chemical Society 121 (14): 3543–3544. doi:10.1021/ja9844229. ISSN 0002-7863.
- ↑ Lee, Kwangho; Cha, Jin Kun (2001-06-01). "Formal Synthesis of (+)-Phorbol1". Journal of the American Chemical Society 123 (23): 5590–5591. doi:10.1021/ja010643u. ISSN 0002-7863. PMID 11389648.
- ↑ Trost, Barry M.; Thiel, Oliver R.; Tsui, Hon-Chung (2002-10-01). "DYKAT of Baylis−Hillman Adducts: Concise Total Synthesis of Furaquinocin E". Journal of the American Chemical Society 124 (39): 11616–11617. doi:10.1021/ja0277834. ISSN 0002-7863. PMID 12296725.
- ↑ Ichikawa, Masaya; Takahashi, Masaki; Aoyagi, Sakae; Kibayashi, Chihiro (2004-12-01). "Total Synthesis of (−)-Incarvilline, (+)-Incarvine C, and (−)-Incarvillateine". Journal of the American Chemical Society 126 (50): 16553–16558. doi:10.1021/ja0401702. ISSN 0002-7863. PMID 15600360.
- ↑ Dounay, Amy B.; Humphreys, Philip G.; Overman, Larry E.; Wrobleski, Aaron D. (2008-04-01). "Total Synthesis of the Strychnos Alkaloid (+)-Minfiensine: Tandem Enantioselective Intramolecular Heck−Iminium Ion Cyclization". Journal of the American Chemical Society 130 (15): 5368–5377. doi:10.1021/ja800163v. ISSN 0002-7863. PMID 18303837.
- ↑ Gao, Peng; Cook, Silas P. (2012-07-06). "A Reductive-Heck Approach to the Hydroazulene Ring System: A Formal Synthesis of the Englerins". Organic Letters 14 (13): 3340–3343. doi:10.1021/ol3013167. ISSN 1523-7060. PMID 22679931.
- ↑ Chen, Ji-Qiang; Xie, Jian-Hua; Bao, Deng-Hui; Liu, Sheng; Zhou, Qi-Lin (2012-06-01). "Total Synthesis of (−)-Galanthamine and (−)-Lycoramine via Catalytic Asymmetric Hydrogenation and Intramolecular Reductive Heck Cyclization". Organic Letters 14 (11): 2714–2717. doi:10.1021/ol300913g. ISSN 1523-7060. PMID 22612349.
- ↑ Baran, Phil S.; Maimone, Thomas J.; Richter, Jeremy M. (March 2007). "Total synthesis of marine natural products without using protecting groups". Nature 446 (7134): 404–408. doi:10.1038/nature05569. ISSN 0028-0836. PMID 17377577. Bibcode: 2007Natur.446..404B.
- ↑ Maimone, Thomas J.; Ishihara, Yoshihiro; Baran, Phil S. (June 2015). "Scalable total syntheses of (−)-hapalindole U and (+)-ambiguine H". Tetrahedron 71 (22): 3652–3665. doi:10.1016/j.tet.2014.11.010. ISSN 0040-4020. PMID 25983347.
- ↑ Diethelm, Stefan; Carreira, Erick M. (2013-06-12). "Total Synthesis of (±)-Gelsemoxonine". Journal of the American Chemical Society 135 (23): 8500–8503. doi:10.1021/ja403823n. ISSN 0002-7863. PMID 23688190.
- ↑ Cacchi, S.; Arcadi, A. (November 1983). "Palladium-catalyzed conjugate addition reaction of aryl iodides with .alpha.,.beta.-unsaturated ketones". The Journal of Organic Chemistry 48 (23): 4236–4240. doi:10.1021/jo00171a016. ISSN 0022-3263.
- ↑ Cacchi, S.; Palmieri, G. (1984). "A One-Pot Palladium-Catalyzed Synthesis of β,β-Diarylketones and Aldehydes from Aryl Iodides and α,β-Unsaturated Carbonyl Compounds". Synthesis 1984 (7): 575–577. doi:10.1055/s-1984-30896. ISSN 0039-7881.
- ↑ Cacchi, S.; Palmieri, G. (February 1985). "The palladium-catalyzed conjugate addition type reaction of 2-bromo-arylmercury compounds and 2-bromo-aryl iodides with α,β-enones: A new entry to 1-indanols". Journal of Organometallic Chemistry 282 (1): C3–C6. doi:10.1016/0022-328x(85)87153-9. ISSN 0022-328X.
- ↑ Cacchi, S. (1984). "The palladium-catalyzed conjugate addition type reaction of aryl iodides with α,β-unsaturated ketones". The Journal of Organometallic Chemistry 286: C48–C51. doi:10.1016/0022-328X(84)80252-1.
- ↑ Arcadi, A.; Marinelli, F.; Cacchi, S. (1986-09-23). "The reaction of aryl iodides with hindered α,β,γ,δ-dienones in the presence of the [Pd(OAc)2(PPh3)2]-trialkylammonium formate reagent". Journal of Organometallic Chemistry 312 (2): c27–c32. doi:10.1016/0022-328X(86)80306-0. ISSN 0022-328X.
- ↑ Amorese, A.; Arcadi, A.; Bernocchi, E.; Cacchi, S.; Cerrini, S.; Fedeli, W.; Ortar, G. (January 1989). "Conjugate addition vs. vinylic substitution in palladium-catalysed reaction of aryl halides with β-substituted-α,β-enones and -enals". Tetrahedron 45 (3): 813–828. doi:10.1016/0040-4020(89)80112-7. ISSN 0040-4020.
- ↑ Arcadi, Antonio; Bernocchi, Emilia; Cacchi, Sandro; Marinelli, Fabio (1991). "Palladium-Catalyzed Conjugate Reduction of α, β-Unsaturated Carbonyl Compounds with Potassium Formate". Synlett 1991 (1): 27–28. doi:10.1055/s-1991-20615. ISSN 0936-5214.
- ↑ Raoufmoghaddam, Saeed; Mannathan, Subramaniyan; Minnaard, Adriaan J.; de Vries, Johannes G.; Reek, Joost N. H. (2015-11-12). "Palladium(0)/NHC-Catalyzed Reductive Heck Reaction of Enones: A Detailed Mechanistic Study". Chemistry - A European Journal 21 (51): 18811–18820. doi:10.1002/chem.201503217. ISSN 0947-6539. PMID 26561034. https://dare.uva.nl/personal/pure/en/publications/palladium0nhccatalyzed-reductive-heck-reaction-of-enones-a-detailed-mechanistic-study(f1f32d8a-0e13-4567-804d-b22af8b35678).html.
- ↑ Raoufmoghaddam, Saeed; Mannathan, Subramaniyan; Minnaard, Adriaan J.; de Vries, Johannes G.; de Bruin, Bas; Reek, Joost N. H. (2017-11-30). "Importance of the Reducing Agent in Direct Reductive Heck Reactions". ChemCatChem 10 (1): 266–272. doi:10.1002/cctc.201701206. ISSN 1867-3880. https://pure.uva.nl/ws/files/32226058/Raoufmoghaddam_et_al_2018_ChemCatChem.pdf.
- ↑ Mannathan, Subramaniyan; Raoufmoghaddam, Saeed; Reek, Joost N. H.; de Vries, Johannes G.; Minnaard, Adriaan J. (2016-07-06). "Corrigendum: Palladium(II) Acetate Catalyzed Reductive Heck Reaction of Enones; A Practical Approach". ChemCatChem 8 (15): 2572. doi:10.1002/cctc.201600726. ISSN 1867-3880.
- ↑ McCrindle, Robert; Ferguson, George; Arsenault, Gilles J.; McAlees, Alan J. (1983). "Reaction of tertiary amines with bis(benzonitrile)dichloropalladium(II). Formation and crystal structure analysis of di-µ-chloro-dichlorobis[2-(N,N-di-isopropyliminio)ethyl-C]dipalladium(II)". Journal of the Chemical Society, Chemical Communications (10): 571–572. doi:10.1039/c39830000571. ISSN 0022-4936.
- ↑ Grushin, Vladimir V.; Alper, Howard (May 1993). "Alkali-induced disproportionation of palladium(II) tertiary phosphine complexes, [L2PdCl2], to LO and palladium(O). Key intermediates in the biphasic carbonylation of ArX catalyzed by [L2PdCl2]". Organometallics 12 (5): 1890–1901. doi:10.1021/om00029a052. ISSN 0276-7333.
- ↑ Trzeciak, Anna M.; Ziółkowski, Józef J. (January 2002). "Synthesis of Palladium Benzyl Complexes from the Reaction of PdCl2[P(OPh)3]2 with Benzyl Bromide and Triethylamine: Important Intermediates in Catalytic Carbonylation". Organometallics 21 (1): 132–137. doi:10.1021/om010541c. ISSN 0276-7333.
- ↑ Lu, Connie C.; Peters, Jonas C. (December 2004). "Synthetic, Structural, and Mechanistic Aspects of an Amine Activation Process Mediated at a Zwitterionic Pd(II) Center". Journal of the American Chemical Society 126 (48): 15818–15832. doi:10.1021/ja046415s. ISSN 0002-7863. PMID 15571407. https://authors.library.caltech.edu/47583/8/ja046415ssi20040831_031704.pdf.
- ↑ Amatore, Christian; El Kaïm, Laurent; Grimaud, Laurence; Jutand, Anny; Meignié, Alice; Romanov, Georgy (2014-06-25). "Kinetic Data on the Synergetic Role of Amines and Water in the Reduction of Phosphine-Ligated Palladium(II) to Palladium(0)". European Journal of Organic Chemistry 2014 (22): 4709–4713. doi:10.1002/ejoc.201402519. ISSN 1434-193X.
- ↑ Gottumukkala, Aditya L.; de Vries, Johannes G.; Minnaard, Adriaan J. (2011-02-08). "Pd–NHC Catalyzed Conjugate Addition versus the Mizoroki–Heck Reaction". Chemistry – A European Journal 17 (11): 3091–3095. doi:10.1002/chem.201003643. ISSN 0947-6539. PMID 21305628. https://pure.rug.nl/ws/files/10734318/2011ChemEurJGottumukkalaSupp.pdf.
- ↑ Parveen, Naziya; Saha, Rajib; Sekar, Govindasamy (2017-11-07). "Stable and Reusable Palladium Nanoparticles-Catalyzed Conjugate Addition of Aryl Iodides to Enones: Route to Reductive Heck Products". Advanced Synthesis & Catalysis 359 (21): 3741–3751. doi:10.1002/adsc.201700823. ISSN 1615-4150.
- ↑ 84.0 84.1 Kulagowski, Janusz J.; Curtis, Neil R.; Swain, Christopher J.; Williams, Brian J. (March 2001). "Stereocontrolled Syntheses of Epimeric 3-Aryl-6-phenyl-1-oxa-7-azaspiro[4.5]decane NK-1 Receptor Antagonist Precursors". Organic Letters 3 (5): 667–670. doi:10.1021/ol006944a. ISSN 1523-7060. PMID 11259032.
- ↑ Merlic, Craig A.; Semmelhack, M.F. (July 1990). "An interesting chloride ion effect in the Heck reaction". Journal of Organometallic Chemistry 391 (2): C23–C27. doi:10.1016/0022-328x(90)80183-z. ISSN 0022-328X.
- ↑ 86.0 86.1 Gurak, John A.; Engle, Keary M. (2018-08-24). "Practical Intermolecular Hydroarylation of Diverse Alkenes via Reductive Heck Coupling". ACS Catalysis 8 (10): 8987–8992. doi:10.1021/acscatal.8b02717. ISSN 2155-5435. PMID 30393575.
- ↑ Wang, Chengdong; Xiao, Guanlin; Guo, Tao; Ding, Yalan; Wu, Xiaojin; Loh, Teck-Peng (2018-06-20). "Palladium-Catalyzed Regiocontrollable Reductive Heck Reaction of Unactivated Aliphatic Alkenes". Journal of the American Chemical Society 140 (30): 9332–9336. doi:10.1021/jacs.8b03619. ISSN 0002-7863. PMID 29925236.
- ↑ Li, Jun; Eastgate, Martin D. (2019). "Making better decisions during synthetic route design: leveraging prediction to achieve greenness-by-design". Reaction Chemistry & Engineering 4 (9): 1595–1607. doi:10.1039/c9re00019d. ISSN 2058-9883. https://semanticscholar.org/paper/da8b44994d065ff9123ca44296c193a11a427a9e.
- ↑ Li, Jun; Eastgate, Martin D. (2015). "Current complexity: a tool for assessing the complexity of organic molecules". Organic & Biomolecular Chemistry 13 (26): 7164–7176. doi:10.1039/c5ob00709g. ISSN 1477-0520. PMID 25962620. https://semanticscholar.org/paper/5db35455cd4a147aace4baad9c41dbb2005b0471.
- ↑ Eastgate, Martin D.; Schmidt, Michael A.; Fandrick, Keith R. (2017-02-08). "On the design of complex drug candidate syntheses in the pharmaceutical industry". Nature Reviews Chemistry 1 (2): 0016. doi:10.1038/s41570-017-0016. ISSN 2397-3358.
- ↑ Patil, Siddappa A.; Patil, Renukadevi; Patil, Shivaputra A. (September 2017). "Recent developments in biological activities of indanones". European Journal of Medicinal Chemistry 138: 182–198. doi:10.1016/j.ejmech.2017.06.032. ISSN 0223-5234. PMID 28667874.
- ↑ Kaur, Maninder; Singh, Manjinder; Chadha, Navriti; Silakari, Om (November 2016). "ChemInform Abstract: Oxindole: A Chemical Prism Carrying Plethora of Therapeutic Benefits". ChemInform 47 (47). doi:10.1002/chin.201647285. ISSN 0931-7597.
- ↑ Stokker, Gerald E (January 1987). "Palladium catalyzed stereospecific Michael arylation of 6-alkyl-5,6-dihydro-2H-pyran-2-ones". Tetrahedron Letters 28 (28): 3179–3182. doi:10.1016/s0040-4039(00)95465-0. ISSN 0040-4039.
- ↑ Veenstra, Siem J.; Hauser, Kathleen; Betschart, Claudia (February 1997). "Studies on the active conformation of NK1 antagonist CGP 49823. Part 1. Synthesis of conformationally restricted analogs.". Bioorganic & Medicinal Chemistry Letters 7 (3): 347–350. doi:10.1016/s0960-894x(96)00623-3. ISSN 0960-894X.
- ↑ Tobrman, Tomáš; Dvořák, Dalimil (January 2004). "'Reductive Heck reaction' of 6-halopurines". Tetrahedron Letters 45 (2): 273–276. doi:10.1016/j.tetlet.2003.10.181. ISSN 0040-4039.
- ↑ Göksu, Gökce; Gül, Melek; Öcal, Nüket; Kaufmann, Dieter E. (April 2008). "Hydroarylation of bicyclic, unsaturated dicarboximides: access to aryl-substituted, bridged perhydroisoindoles". Tetrahedron Letters 49 (17): 2685–2688. doi:10.1016/j.tetlet.2008.02.171. ISSN 0040-4039.
- ↑ Li, Ze; Watkins, E. Blake; Liu, Hua; Chittiboyina, Amar G.; Carvalho, Paulo B.; Avery, Mitchell A. (2008-10-03). "1,3-Diaxially Substituted trans-Decalins: Potential Nonsteroidal Human Progesterone Receptor Inhibitors". The Journal of Organic Chemistry 73 (19): 7764–7767. doi:10.1021/jo800947m. ISSN 0022-3263. PMID 18771323.
- ↑ Goksu, Gokce; Ocal, Nuket; Kaufmann, Dieter E. (2010-03-04). "Reductive Heck Reactions of N-Methyl-substituted Tricyclic Imides". Molecules 15 (3): 1302–1308. doi:10.3390/molecules15031303. ISSN 1420-3049. PMID 20335982.
- ↑ Gunkara, Omer; Sucu, Bilgesu; Ocal, Nuket; Kaufmann, Dieter (2013-01-01). "Synthesis of new aryl(hetaryl)-substituted tandospirone analogues with potential anxiolytic activity via reductive Heck type hydroarylations". Chemical Papers 67 (6). doi:10.2478/s11696-013-0338-4. ISSN 1336-9075.
- ↑ Sweeney, Joseph B.; Doulcet, Julien; Thapa, Bimod (November 2018). "Synthesis of 3-Substituted Pyrrolidines via Palladium-Catalyzed Hydroarylation". iScience 9: 328–336. doi:10.1016/j.isci.2018.10.025. ISSN 2589-0042. PMID 30448732. Bibcode: 2018iSci....9..328S.