Chemistry:Modafinil sulfone
| |
| Names | |
|---|---|
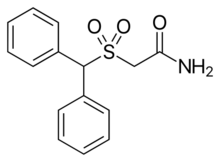
| Preferred IUPAC name
2-(Diphenylmethanesulfonyl)acetamide | |
| Other names
CRL-41056; Diphenylmethylsulfonylacetamide
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Modafinil sulfone (code name CRL-41056) is an achiral, oxidized metabolite of modafinil, a wakefulness-promoting agent. It is one of two major circulating metabolites of modafinil, the other being modafinil acid. Modafinil sulfone is also a metabolite of the modafinil prodrug, adrafinil. Modafinil sulfone is also a metabolite of armodafinil, the (R)-(–)-enantiomer of modafinil, as oxidation to the sulfone removes the chiral center at the sulfur atom. Modafinil sulfone has been described as inactive,[1] and similarly to modafinil acid, does not appear to contribute to the wakefulness-promoting effects of modafinil.[2][3][4] However, like modafinil, modafinil sulfone was found to show anticonvulsant properties in animals, indicating that it does possess some biological activity.[5][6]
References
- ↑ Wong, Y. Nancy; Wang, Lixia; Hartman, Linda; Simcoe, Donna; Chen, Yusong; Laughton, Watson; Eldon, Richard; Markland, Colin et al. (1998). "Comparison of the Single-Dose Pharmacokinetics and Tolerability of Modafinil and Dextroamphetamine Administered Alone or in Combination in Healthy Male Volunteers". The Journal of Clinical Pharmacology 38 (10): 971–978. doi:10.1002/j.1552-4604.1998.tb04395.x. ISSN 0091-2700. PMID 9807980.
- ↑ Schwertner, Harvey A.; Kong, Suk Bin (2005). "Determination of modafinil in plasma and urine by reversed phase high-performance liquid-chromatography". Journal of Pharmaceutical and Biomedical Analysis 37 (3): 475–479. doi:10.1016/j.jpba.2004.11.014. ISSN 0731-7085. PMID 15740906. https://zenodo.org/record/1259161.
- ↑ Robertson, Philmore; Hellriegel, Edward T. (2003). "Clinical Pharmacokinetic Profile of Modafinil". Clinical Pharmacokinetics 42 (2): 123–137. doi:10.2165/00003088-200342020-00002. ISSN 0312-5963. PMID 12537513.
- ↑ Robertson, P (2002). "Effect of modafinil on the pharmacokinetics of ethinyl estradiol and triazolam in healthy volunteers". Clinical Pharmacology & Therapeutics 71 (1): 46–56. doi:10.1067/mcp.2002.121217. ISSN 0009-9236. PMID 11823757.
- ↑ "Anti-narcoleptic agent modafinil and its sulfone: a novel facile synthesis and potential anti-epileptic activity". Neurochem. Res. 29 (8): 1481–6. 2004. doi:10.1023/b:nere.0000029559.20581.1a. PMID 15260124.
- ↑ "Modafinil side effects". https://www.modafinilonline.org/. Monday, 7 January 2019
 |

