Chemistry:N-Sulfinyl amine
From HandWiki
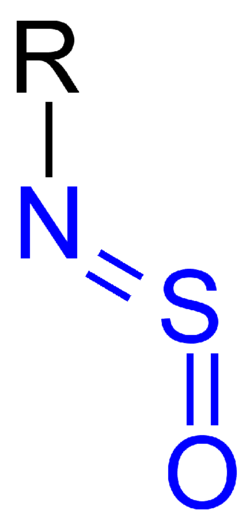
An N-sulfinyl amine is an organic group with formula -NSO.[1] There is a double bond between the sulfur and oxygen. N-sulfinyl amine is like sulfur dioxide with one oxygen replaced by nitrogen, or isocyanate, with carbon replaced by sulfur. This group has a bent structure.
Another type of sulfinyl amine has a structure: RN(H)S(O)R" or RR'NS(O)R".[2]
It can be contrasted with sulfonylamino- compounds, with two oxygen atoms on the sulfur.
Preparation
N-sulfinyl amines can be made when thionyl chloride SOCl2 reacts with a primary amine.[3]
Reactions
Mixtures of phosphine and borane derivatives can attach to the NSO chain to yield a R'3P=N+(R)SOB−R"3 compound. This can happen with tris(tert-butyl) phosphine and tris-(pentafluorophenyl)borane.[3]
Compounds
| formula | name | CAS No | PubChem CID | Chemspider ID | MW | ref |
|---|---|---|---|---|---|---|
| HNSO | Thionylimide Sulfinylamine Sulfoximine |
13817-04-4 | 139610 | 123125 | 63.074 | [4] |
| C6H5NSO | N-Sulfinylaniline N-Thionylaniline |
1122-83-4 | 70739 | 63904 | 139.172 | [5] |
| N-sulfinyl-2,6-diethyl benzenamine | [5] | |||||
| N-sulfinyl-2-aminopyrimidine | 110526-12-0 | 14790782 | 141.148 | |||
| N-sulfinyl-n-butylamine | [6] | |||||
| N-sulfinyl-n-pentylamine | [6] |
References
- ↑ (in en) Krishna's Advanced Organic Chemistry; Volume 1. Krishna Prakashan Media. p. 289. ISBN 9788182830783. https://books.google.com/books?id=AH6vVI4C3PMC&pg=PA289.
- ↑ Chen, Wen; Ren, Jian; Wang, Minshou; Dang, Lingjing; Shen, Xianfu; Yang, Xiaodong; Zhang, Hongbin (2014). "Iodine mediated deprotection of N-tert-butanesulfinyl amines: a functional group compatible method". Chem. Commun. 50 (47): 6259–6262. doi:10.1039/C4CC00958D. PMID 24788957.
- ↑ Jump up to: 3.0 3.1 Longobardi, Lauren E.; Wolter, Vanessa; Stephan, Douglas W. (12 January 2015). "Frustrated Lewis Pair Activation of an N-Sulfinylamine: A Source of Sulfur Monoxide". Angewandte Chemie International Edition 54 (3): 809–812. doi:10.1002/anie.201409969. PMID 25376102.
- ↑ Kresze, G.; Maschke, A.; Albrecht, R.; Bederke, K.; Patzschke, H. P.; Smalla, H.; Trede, A. (February 1962). "Organic N-Sulfinyl Compounds". Angewandte Chemie International Edition in English 1 (2): 89–98. doi:10.1002/anie.196200891.
- ↑ Jump up to: 5.0 5.1 Romano, R.M; Della Védova, C.O; Boese, R (January 1999). "A solid state study of the configuration and conformation of OSN–R (R=C6H5 and C6H3(CH3–CH2)2-2,6)". Journal of Molecular Structure 475 (1): 1–4. doi:10.1016/S0022-2860(98)00439-6.
- ↑ Jump up to: 6.0 6.1 Ammoscato, Vince (1990). "Part I. A study of the alkylation chemistry of N-sulfinyl amines. Part II. Attempted preparation of the camphor imine of stereospecifically deuterated glycine." (in en). University of Windsor. https://scholar.uwindsor.ca/etd/3002/. Retrieved 28 January 2018.