Chemistry:Neber rearrangement
From HandWiki
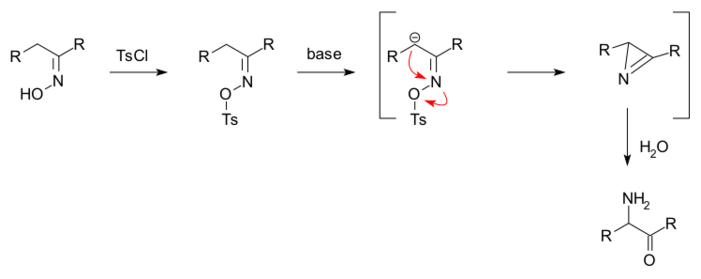
The Neber rearrangement is an organic reaction in which a ketoxime is converted into an alpha-aminoketone via a rearrangement reaction.[1][2][3]
The oxime is first converted to an O-sulfonate, for example a tosylate by reaction with tosyl chloride. Added base forms a carbanion which displaces the tosylate group in a nucleophilic displacement to an azirine and added water subsequently hydrolyses it to the aminoketone.
The Beckmann rearrangement is a side reaction.[4]
References
- ↑ March, Jerry (1985), Advanced Organic Chemistry: Reactions, Mechanisms, and Structure (3rd ed.), New York: Wiley, ISBN 0-471-85472-7
- ↑ P. W. Neber and A. v. Friedolsheim (1926). "Über eine neue Art der Umlagerung von Oximen". Justus Liebig's Annalen der Chemie 449 (1): 109–134. doi:10.1002/jlac.19264490108.
- ↑ O'Brien, Connor (1 April 1964). "The Rearrangement of Ketoxime O-Sulfonates to Amino Ketones (The Neber Rearrangement)". Chemical Reviews 64 (2): 81–89. doi:10.1021/cr60228a001.
- ↑ Uyanik, M.; Ishihara, K. (2014-01-01), Knochel, Paul, ed., "6.14 Functional Group Transformations via Carbonyl Derivatives" (in en), Comprehensive Organic Synthesis (Second Edition) (Amsterdam: Elsevier): pp. 573–597, ISBN 978-0-08-097743-0, https://www.sciencedirect.com/science/article/pii/B9780080977423006224, retrieved 2022-10-10
 |