Chemistry:Nonaflate
From HandWiki
This article needs additional citations for verification. (December 2006) (Learn how and when to remove this template message) |
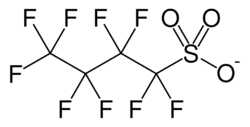
Skeletal formula of the nonaflate anion
Nonaflate, CF3CF2CF2CF2SO−3, is the common name given to nonafluorobutanesulfonates, the salts or esters of perfluorobutanesulfonic acid. Its uses are similar to those of triflate. It is a good leaving group.[1] It is a substitute for more toxic long-chain PFAS chemicals.[2]
References
- ↑ Smith, Michael B.; March, Jerry (2007). March's Advanced Organic Chemistry (6th ed.). John Wiley & Sons. p. 497. ISBN 978-0-471-72091-1.
- ↑ Thurlow, Matthew (December 27, 2018). "Fear and loathing of PFAS". American Bar Association. https://www.americanbar.org/groups/environment_energy_resources/publications/trends/2018-2019/january-february-2019/fear-and-loathing/.
 |

