Chemistry:Oddo–Harkins rule
The Oddo–Harkins rule holds that an element with an even atomic number is more abundant than the elements with immediately adjacent atomic numbers. For example, carbon, with atomic number 6, is more abundant than boron (5) and nitrogen (7). This pattern was first reported by Giuseppe Oddo[1] in 1914 and William Draper Harkins[2] in 1917.[3]
Definition
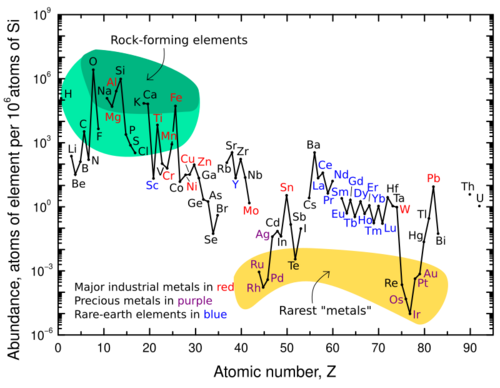
All atoms bigger than hydrogen are formed in stars or supernovae through nucleosynthesis, when gravity, temperature and pressure reach levels high enough to fuse protons and neutrons together. Protons and neutrons form the atomic nucleus, which accumulates electrons to form atoms. The number of protons in the nucleus, called atomic number, uniquely identifies a chemical element.
The Oddo–Harkins rule argues that elements with odd atomic numbers have one unpaired proton and so are more likely to capture another, increasing their atomic number. It is possible that in elements with even atomic numbers, protons are paired, with each member of the pair balancing the spin of the other; even parity thus enhances nucleon stability.
Exceptions to the rule
This postulate, however, does not apply to the universe's most abundant and simplest element: hydrogen, with an atomic number of 1. This may be because, in its ionized form, a hydrogen atom becomes a single proton, of which it is theorized to have been one of the first major conglomerates of quarks during the initial second of the Universe's inflation period, following the Big Bang. In this period, when inflation of the universe had brought it from an infinitesimal point to about the size of a modern galaxy, temperatures in the particle soup fell from over a trillion degrees to several million degrees.
This period allowed for the fusion of single protons and deuterium nuclei to form helium and lithium nuclei but was too short for every H+ ion to be reconstituted into heavier elements. In this case, helium, atomic number 2, remains the even numbered counterpart to hydrogen. Thus, neutral hydrogen—or hydrogen paired with an electron, the only stable lepton—constituted the vast majority of the remaining unannihilated portions of matter following the conclusion of inflation.
Another exception to the rule is beryllium which, despite an even atomic number (4), is rarer than adjacent elements (lithium and boron). This is because most of the universe's lithium, beryllium, and boron are made by cosmic ray spallation, not ordinary stellar nucleosynthesis, and beryllium has only one stable isotope, causing it to lag in abundance with regard to its neighbors, each of which has two stable isotopes.
Relationship to fusion
Depending on the mass of a star, the Oddo-Harkins pattern arises from the burning of progressively more massive elements within a collapsing dying star by fusion processes such as the proton-proton chain, the CNO cycle, and the triple-alpha process. The newly formed elements are ejected slowly via stellar wind, or in the explosion of a supernova, and will eventually join the rest of the galaxy's interstellar medium.
See also
- Chemistry:List of elements by stability of isotopes – None
- Chemistry:Nuclear chemistry – Branch of chemistry dealing with radioactivity, transmutation and other nuclear processes
References
- ↑ Oddo, Giuseppe (1914). "Die Molekularstruktur der radioaktiven Atome". Zeitschrift für Anorganische Chemie 87: 253–268. doi:10.1002/zaac.19140870118. https://zenodo.org/record/1428150.
- ↑ Harkins, William D. (1917). "The Evolution of the Elements and the Stability of Complex Atoms". Journal of the American Chemical Society 39 (5): 856–879. doi:10.1021/ja02250a002. https://zenodo.org/record/1429060.
- ↑ North, John (2008). Cosmos an illustrated history of astronomy and cosmology (Rev. and updated ed.). Univ. of Chicago Press. pp. 602. ISBN 978-0-226-59441-5. https://books.google.com/books?id=qq8Luhs7rTUC&q=%22william+draper+harkins%22+oddo&pg=PA602.
 |


