Chemistry:Rieche formylation
From HandWiki
Short description: Chemical reaction
| Rieche formylation | |
|---|---|
| Named after | Alfred Rieche |
| Reaction type | Substitution reaction |
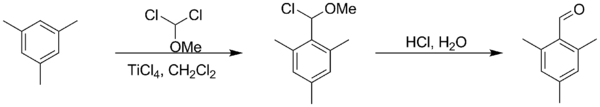
Rieche formylation is a type of formylation reaction. The substrates are electron rich aromatic compounds, such as mesitylene[1] or phenols,[2] with dichloromethyl methyl ether acting as the formyl source. The catalyst is titanium tetrachloride and the workup is acidic. The reaction is named after Alfred Rieche who discovered it in 1960.[3]
See also
References
- ↑ Rieche, A.; Gross, H.; Höft, E. (1967). "Aromatic Aldehydes. Mesitaldehyde". Organic Syntheses 47 (47): 1. doi:10.15227/orgsyn.047.0001.
- ↑ García, Oscar; Nicolás, Ernesto; Albericio, Fernando (June 2003). "o-Formylation of electron-rich phenols with dichloromethyl methyl ether and TiCl4". Tetrahedron Letters 44 (27): 4961–4963. doi:10.1016/S0040-4039(03)01168-7.
- ↑ Rieche, Alfred; Gross, Hans; Höft, Eugen (January 1960). "Über α-Halogenäther, IV. Synthesen aromatischer Aldehyde mit Dichlormethyl-alkyläthern". Chemische Berichte 93 (1): 88–94. doi:10.1002/cber.19600930115.
 |