Chemistry:Secalonic acid
Secalonic acids are a group of xanthone derivatives closely related to ergoflavin and ergochrysin A that are collectively called ergochromes and belong to a class of mycotoxins initially isolated as major ergot pigments from the fungi Claviceps purpurea that grows parasitically on rye grasses.[1][2] From early times and particularly in medieval Europe the consumption of grains containing ergot has repeatedly lead to mass poisonings known as ergotism which was caused by toxic ergot alkaloids and mycotoxins such as the ergochromes, due to contamination of flour by C. purpurea. A cluster of genes responsible for the synthesis of secalonic acids in C. purpurea has been identified.[3] Secalonic acid D the enantiomer of secalonic acid A is a major environmental toxin, isolated from the fungus Penicillium oxalicum, and is a major microbial contaminant of freshly-harvested corn which causes toxicity through contamination of foodstuffs.[1][2]
- Major Ergot Ergochromes and Secalonic acid D
-
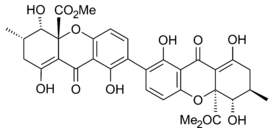
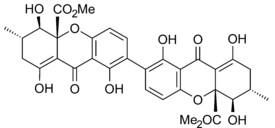
Secalonic acid A
-
Secalonic acid B
-
Secalonic acid C
-
Secalonic acid D
-
Ergoflavin
-
Ergochrysin A
Occurrence
In addition to the occurrence in C. purpurea the secalonic acids A, B, D and ergoflavin have also been isolated from other fungi, and the three secalonic acids have also been found in various lichens.[1] To date at least twenty-two members of the ergochrome family have been isolated and structurally identified,[4] including secalonic acid E (the enantiomer of secalonic acid A) from the fungus Phoma terrestris, secalonic acid F from the fungus Aspergillus aculeatus, and secalonic acid G from the fungus Pyrenochaeta terrestris.[1] In addition the monomeric units of the dimeric secalonic acids, namely hemisecalonic acids B, and E (blennolides A and E) have been isolated from Blennoria sp., an endophytic fungus from Carpobrotus edulis.[4]
Bioactivity
The secalonic family of secondary metabolite mycotoxins exhibit interesting bioactivities. Secalonic acid A has antitumor properties and also reduces colchicines toxicity in rat cortical neurons.[5] In addition, it has been demonstrated that secalonic acid A protects against dopaminergic neuron death in a Parkinson's disease mouse model.[6] Secalonic acid B also has antitumor activity. When tested against B16 murine melanoma it was found to be active in the low micromolar range.[7] It also proved to be an effective antimicrobial agent against the Gram-positive bacteria (Bacillus megaterium) and the Gram-negative bacteria (Escherichia coli) and was found to be antifungal against (Microbotryum violaceum) and antialgal against (Chlorella fusca).[4] Secalonic acid D (SAD) is a toxic and teratogenic metabolite. Teratogenic effects were observed in the development of rats that were exposed to SAD injected during fetal development.[1] SAD exhibited potent cytotoxicity on multidrug resistance (MDR) cells and their parental cells. Investigation of the antitumor activity of SAD showed that it exerted potent cytotoxic activity on SP cells, due to induction of ABCG2 degradation by calpain-1 activation.[8] Ergoflavin showed good anti-inflammatory activity and good anticancer activities including significant inhibition of proliferation particularly in pancreatic, renal, and lung cancer cells,[9] and may be exerting its effects via mechanisms similar to those of secalonic acid D.
Structure
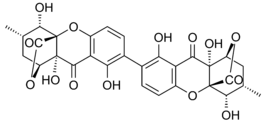
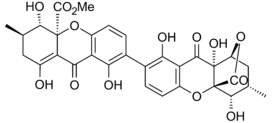
Ergoflavin was first isolated in pure form from Claviceps purpurea (ergot) in 1958.[10] It was shown to be a 2,2’- biaryl linked dimer in 1963 and the structure confirmed that year by single-crystal X-ray analysis.[1] During the following decade the structures of secalonic acids A, B, C, D and ergochrysin A were similarly firmly established,[11] and although there was some early contention whether they were 2,2’-, 4,4’- or even 2,4’-linked [1] it was confirmed that they too were all 2,2’- linked between the biphenyl residues.[12] In all known secalonic acids, the methyl and methoxycarbonyl substituents are found to be trans to each other, and X-ray analysis of the crystal structure of secalonic acid A showed that the 2,2’-biaryl linkage was nonplanar and the angle between the two biphenyl planes was 36.5°.[1]

The tetrahydroxanthone-containing secalonic acids have been demonstrated to be unstable under basic conditions, and they can easily undergo isomerizations arising from ether linkage replacement.[1] The 2-2’linked secalonic acid A isomerizes in DMSO at room temperature to the 2-4’linked secalonic acid A and 4-4’linked secalonic acid A during 13hr, to reach an equilibrium of 3.2 : 2 : 1.[13] This isomerisation goes faster in the presence of base (DMSO/pyridine).
Synthesis
The common key feature in the synthesis of ergoflavin and the secalonic acids is the biaryl dimerisation of protected iodo-aryl monomers with Cu or Pd. Whalley's synthesis of ergoflavin 3 from hemiergoflavin 1 in 1971 was achieved by a low yield coupling of two protected 2-iodo-hemiegoflavin monomers 2 with copper under the Ullmann reaction conditions, followed by acid deprotection.[11][12]

Similarly more than forty years later Porco's synthesis of the more labile secalonic acid D in 60% yield involved coupling two protected iodo monomers via their stannes with CuCl at room temperature,[14] whereas Tietze achieved a similar synthesis of secalonic acid E by coupling two protected iodo monomers with Pd (OAc)2under Suzuki conditions at 70 °C in 85% yield.[15]


References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 1.7 1.8 Masters KS; Bräse S (May 2012). "Xanthones from fungi, lichens, and bacteria: the natural products and their synthesis". Chemical Reviews 112 (7): 3717–3776. doi:10.1021/cr100446h. PMID 22617028.
- ↑ 2.0 2.1 "Xanthone dimers: a compound family which is both common and privileged". Natural Product Reports 32 (1): 6–28. Jan 2015. doi:10.1039/c4np00050a. PMID 25226564. https://publikationen.bibliothek.kit.edu/1000046415/3466827.
- ↑ Neubauer, Lisa; Dopstadt, Julian; Humpf, Hans-Ulrich; Tudzynski, Paul (2016). "Identification and characterization of the ergochrome gene cluster in the plant pathogenic fungus Claviceps purpurea". Fungal Biology and Biotechnology 3: 2. doi:10.1186/s40694-016-0020-z. ISSN 2054-3085. PMID 28955461.
- ↑ 4.0 4.1 4.2 "New Mono‐and Dimeric Members of the Secalonic Acid Family: Blennolides A–G Isolated from the Fungus Blennoria sp.". Chemistry: A European Journal 14 (16): 4913–4923. May 2008. doi:10.1002/chem.200800035. PMID 18425741.
- ↑ "Secalonic acid A reduced colchicine cytotoxicity through suppression of JNK, p38 MAPKs and calcium influx". Neurochemistry International 58 (1): 85–91. January 2011. doi:10.1016/j.neuint.2010.10.016. PMID 21073911.
- ↑ Zhai, Aifeng; Zhu, Xiaonan; Wang, Xuelan; Chen, Ruzhu; Wang, Hai (2013). "Secalonic acid A protects dopaminergic neurons from 1-methyl-4-phenylpyridinium (MPP+)-induced cell death via the mitochondrial apoptotic pathway". European Journal of Pharmacology 713 (1–3): 58–67. doi:10.1016/j.ejphar.2013.04.029. ISSN 0014-2999. PMID 23665112.
- ↑ "Cytotoxic constituents of the lichen Diploicia canescens". Journal of Natural Products 72 (12): 2177–2180. November 2009. doi:10.1021/np9003728. PMID 19919064.
- ↑ Hu, Ya-peng; Tao, Li-yang; Wang, Fang; Zhang, Jian-ye; Liang, Yong-ju; Fu, Li-wu (2013). "Secalonic acid D reduced the percentage of side populations by down-regulating the expression of ABCG2". Biochemical Pharmacology 85 (11): 1619–1625. doi:10.1016/j.bcp.2013.04.003. ISSN 0006-2952. PMID 23583455.
- ↑ "Anti‐inflammatory and anticancer activity of ergoflavin isolated from an endophytic fungus". Chemistry & Biodiversity 6 (5): 784–789. May 2009. doi:10.1002/cbdv.200800103. PMID 19479845.
- ↑ "373. The chemistry of fungi. Part XXXV. A preliminary investigation of ergoflavin". Journal of the Chemical Society (Resumed): 1833–1842. 1958. doi:10.1039/JR9580001833.
- ↑ 11.0 11.1 "The chemistry of fungi. Part LXV. The structures of ergochrysin A, isoergochrysin A, and ergoxanthin, and of secalonic acids A, B, C, and D.". Journal of the Chemical Society C: Organic 21: 3580–3590. 1971. doi:10.1039/J39710003580. PMID 5167268.
- ↑ 12.0 12.1 "The position of the biphenyl linkage in the ergot pigments. A partial synthesis of ergoflavin". Journal of the Chemical Society D: Chemical Communications (2): 111–112. 1971. doi:10.1039/C29710000111.
- ↑ "Syntheses of Dimeric Tetrahydroxanthones with Varied Linkages: Investigation of "Shapeshifting" Properties". Journal of the American Chemical Society 137 (48): 15225–15233. November 2015. doi:10.1021/jacs.5b09825. PMID 26544765.
- ↑ "Total syntheses of secalonic acids A and D". Angewandte Chemie International Edition 53 (12): 3107–3110. March 2014. doi:10.1002/anie.201311260. PMID 24519991.
- ↑ "Enantioselective Total Synthesis of Secalonic Acid E". Chemistry: A European Journal 21 (47): 16807–16810. November 2015. doi:10.1002/chem.201503593. PMID 26447631.
 |