Chemistry:Silanization
Silanization is the covering of a surface with organofunctional alkoxysilane molecules. Mineral components like glass and metal oxide surfaces can all be silanized, because they contain hydroxyl groups which attack and displace the alkoxy groups on the silane thus forming a covalent -Si-O-Si- bond. The goal of silanization is to form bonds across the interface between mineral components and organic components present in paints, adhesives, etc. Silanization (or siliconization) of glassware increases its hydrophobicity and is used in cell culturing to reduce adherence of cells to flask walls.[1]
Properties
Organofunctional alkoxysilane molecules have both organic and inorganic properties.
Mechanism
The mechanism of silanization start from removing hydroxyl group from the surface of the substrate. Then, the surface will attach to any silane group.
Organofunctional alkoxysilanes
The alkoxy groups usually used are the methoxy (-OCH3) and the ethoxy (-OCH2CH3) groups. The organofunctional alkoxysilanes are classified according to their organic functions:
Aminosilanes
The organic function is a primary or secondary amine:
- APTES (3-aminopropyl)-triethoxysilane CAS# 919-30-2
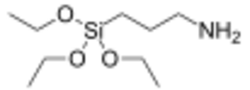
- APDEMS (3-aminopropyl)-diethoxy-methylsilane
- APDMES (3-aminopropyl)-dimethyl-ethoxysilane
- APTMS (3-aminopropyl)-trimethoxysilane CAS# 13822-56-5
Glycidoxysilanes
The organic function is an epoxide:
- GPMES (3-glycidoxypropyl)-dimethyl-ethoxysilane
Mercaptosilanes
The organic function is a thiol:
- MPTMS (3-mercaptopropyl)-trimethoxysilane
- MPDMS (3-mercaptopropyl)-methyl-dimethoxysilane
References
- ↑ Seed, Brian (May 2001). "APPENDIX 3E Silanizing Glassware". Current Protocols in Cell Biology: A.3E.1. doi:10.1002/0471143030.cba03es08.
