Chemistry:Thia-crown ether
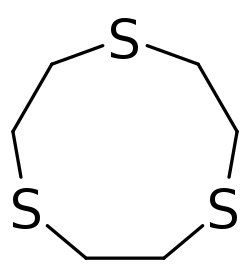
2CH
2S)
3 (9-ane-S3)
In organic chemistry, thia-crown ethers are organosulfur compounds which are the thia analogues of crown ethers (cyclic polyethers). That is, they have a sulfur atom (sulfide linkage, –S–) in place of each oxygen atom (ether linkage, –O–) around the ring. While the parent crown ethers have the formulae (CH
2CH
2O)
n, the parent thia-crown ethers have the formulae (CH
2CH
2S)
n, where n = 3, 4, 5, 6. They have trivial names "x-ane-Sy",[citation needed] where x and y are the number of atoms in the ring and the number of those atoms that are sulfur, respectively. Thia-crown ethers exhibit affinities for transition metals.
1,4,7-Trithiacyclononane (9-ane-S3) is a tridentate ligand and forms complexes with many metal ions, including those considered hard, such as copper(II) and iron(II).[1]
Tetradentate 14-ane-S4[2] and the hexadentate 18-ane-S6[3] are also known.
References
- ↑ Kueppers, H. J.; Wieghardt, K.; Nuber, B.; Weiss, J. W.; Bill, E.; Trautwein, A. X. (1987). "Crown Thioether Chemistry of Iron(II/III). Synthesis and Characterization of Low-spin Bis(1,4,7-trithiacyclononane)iron(III) and crystal structure of [FeII([9]aneS3)([9]aneS3(O))](ClO4)2•2NaClO4•H2O". Inorganic Chemistry 26 (22): 3762–3769(8). doi:10.1021/ic00269a028.
- ↑ Prett, V; Diaddario, L; Dockal, E; Corfield, P; Ceccarelli, C; Glick, M; Ochrymowycz, L. A.; Rorabacher, D. B. (1983). "Ring size effects on the structure of macrocyclic ligand complexes: copper(II) complexes with 12–16-membered cyclic tetrathia ethers". Inorganic Chemistry 22 (24): 3661–3670. doi:10.1021/ic00166a033.
- ↑ Shaw, J; Wolowska, J; Collison, D; Howard, J; McInnes, E; McMaster, J; Blake, A; Wilson, C et al. (2006). "Redox Non-innocence of Thioether Macrocycles: Elucidation of the Electronic Structures of Mononuclear Complexes of Gold(II) and Silver(II)". Journal of the American Chemical Society 128 (42): 13827–13839. doi:10.1021/ja0636439. PMID 17044711.
 |
