Chemistry:Tin mesoporphyrin
| |
| Names | |
|---|---|
| IUPAC name
3-[18-(2-carboxyethyl)-8,13-diethyl-3,7,12,17-tetramethylporphyrin-21,24-diid-2-yl]propanoic acid;tin(4+);dichloride
| |
| Identifiers | |
3D model (JSmol)
|
|
| DrugBank | |
PubChem CID
|
|
| UNII | |
| |
| |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tin mesoporphyrin (SnMP), also known as stannsoporfin, is a synthetic metalloporphyrin,[1] which consists of a group of competitive inhibitors of heme oxygenase, a rate-limiting enzyme in the heme catabolic pathway.[2] Tin mesoporphyrin is one of the more potent metalloporphyrin compound out of all the others.
Structure
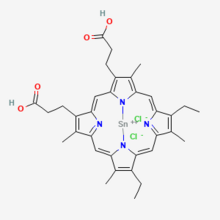
As the name, tin mesoporphyrin, suggests, the overall structure is very similar to the naturally occurring heme molecule. It consists of a ring structured protoporphyrin IX molecule that has tin as its central atom.[3] As this is a synthetic molecule, the two vinyl groups at both the C2 and C4 positions on the porphyrin macrocyle are reduced to form ethyl groups[4] that is found on tin mesoporphyrin. The molecular weight of tin mesoporphyrin is 754.3 g/mol.[5]
Mechanism
In the heme catabolic pathway, heme oxygenase catalyzes the breakdown of heme to biliverdin and well as carbon monoxide that is exhaled. Biliverdin is then converted to bilirubin with biliverdin reductase.[6] As high biliverdin levels are usually related to bilirubinemia, tin mesoporphyrin has been found to aid in treatment and prevention of this, primarily in newborn infants. Tin mesoporphyrin competitively inhibits the heme oxygenase enzyme, which prevents the breakdown of heme to biliverdin leading to accumulation of heme and not bilirubin.[7]
Application
The application of stannsoporfin or tin mesoporphyrin is currently still being researched. Clinical studies have outlined its use in the treatment of hyperbilirubinemia infants[8] and also the prevention of neonatal jaundice. It has also been found to reduce edema and hematoma in spontaneous intracerebral hemorrhage(ICH) from patients who suffered traumatic brain injuries.[9]
Treatment in hyperbilirubinemia
In one study, tin mesoporphyrin was administered intramuscularly to a newborn that was only 46 hours old with a low birth weight who had also suffered from severe hyperbilirubinemia.[10] Along with blue light treatment, the newborn showed a steady decrease in its total serum biliverdin within 10 hours after administration.[11]
Prevention of neonatal jaundice
It has also been found that tin mesoporphyrin can aid in the prevention of neonatal jaundice. When administered to pre-discharge newborns who were at risk for neonatal jaundice the results showed a decrease in total biliverdin load, the possibility of postnatal bilirubin progression, as well as the use and duration of phototherapy.[12]
Treatment in spontaneous intracerebral hemorrhage
Tin mesoporphyrin has also been found potentially reduce intracerebral mass in intracerebral hemorrhage cases by decreasing the hematoma and edema volumes.[13]
Concerns of use
Tin is a metal and can potentially be toxic. Consider the application of it in terms of dosage and other contributing factors. If tin mesoporphyin is going to be utilized in medicine, it is important to understand if there are any specific interactions with other medications. Since this compound has not been thoroughly investigated yet, there may be adverse effects of symptoms that can come with its application.
References
- ↑ "Mesoporphyrin". https://www.sciencedirect.com/topics/medicine-and-dentistry/mesoporphyrin.
- ↑ "Stannsoporfin". https://pubchem.ncbi.nlm.nih.gov/compound/Stannsoporfin.
- ↑ Poudel, P.; Adhikari, S. (2022). "Efficacy and Safety Concerns with Sn-Mesoporphyrin as an Adjunct Therapy in Neonatal Hyperbilirubinemia: A Literature Review". International Journal of Pediatrics 2022: 1–7. doi:10.1155/2022/2549161. PMID 35898803.
- ↑ Sorrenti, Valeria; d'Amico, Agata Grazia; Barbagallo, Ignazio; Consoli, Valeria; Grosso, Salvo; Vanella, Luca (2021). "Tin Mesoporphyrin Selectively Reduces Non-Small-Cell Lung Cancer Cell Line A549 Proliferation by Interfering with Heme Oxygenase and Glutathione Systems". Biomolecules 11 (6): 917. doi:10.3390/biom11060917. PMID 34205698.
- ↑ "Tin-mesoporphyrin". https://pubchem.ncbi.nlm.nih.gov/compound/Tin-mesoporphyrin.
- ↑ Poudel, P.; Adhikari, S. (2022). "Efficacy and Safety Concerns with Sn-Mesoporphyrin as an Adjunct Therapy in Neonatal Hyperbilirubinemia: A Literature Review". International Journal of Pediatrics 2022: 1–7. doi:10.1155/2022/2549161. PMID 35898803.
- ↑ Poudel, P.; Adhikari, S. (2022). "Efficacy and Safety Concerns with Sn-Mesoporphyrin as an Adjunct Therapy in Neonatal Hyperbilirubinemia: A Literature Review". International Journal of Pediatrics 2022: 1–7. doi:10.1155/2022/2549161. PMID 35898803.
- ↑ Reddy, Pradeep; Najundaswamy, Shakuntala; Mehta, Rajeev; Petrova, Anna; Hegyi, Thomas (2003). "Tin-mesoporphyrin in the Treatment of Severe Hyperbilirubinemia in a Very-low-birth-weight Infant". Journal of Perinatology 23 (6): 507–508. doi:10.1038/sj.jp.7210943. PMID 13679941.
- ↑ Wagner, K. R.; Hua, Y.; De Courten-Myers, G. M.; Broderick, J. P.; Nishimura, R. N.; Lu, S. Y.; Dwyer, B. E. (2000). "Tin-mesoporphyrin, a potent heme oxygenase inhibitor, for treatment of intracerebral hemorrhage: in vivo and in vitro studies". Cellular and Molecular Biology (Noisy-Le-Grand, France) 46 (3): 597–608. PMID 10872746. https://pubmed.ncbi.nlm.nih.gov/10872746/#:~:text=In%20cerebral%20ischemia%20models%2C%20metalloporphyrins,used%20clinically%20to%20treat%20hyperbilirubinemia..
- ↑ Reddy, Pradeep; Najundaswamy, Shakuntala; Mehta, Rajeev; Petrova, Anna; Hegyi, Thomas (2003). "Tin-mesoporphyrin in the Treatment of Severe Hyperbilirubinemia in a Very-low-birth-weight Infant". Journal of Perinatology 23 (6): 507–508. doi:10.1038/sj.jp.7210943. PMID 13679941.
- ↑ Reddy, Pradeep; Najundaswamy, Shakuntala; Mehta, Rajeev; Petrova, Anna; Hegyi, Thomas (2003). "Tin-mesoporphyrin in the Treatment of Severe Hyperbilirubinemia in a Very-low-birth-weight Infant". Journal of Perinatology 23 (6): 507–508. doi:10.1038/sj.jp.7210943. PMID 13679941.
- ↑ Bhutani, V. K.; Poland, R.; Meloy, L. D.; Hegyi, T.; Fanaroff, A. A.; Maisels, M. J. (2016). "Clinical trial of tin mesoporphyrin to prevent neonatal hyperbilirubinemia". Journal of Perinatology 36 (7): 533–539. doi:10.1038/jp.2016.22. PMID 26938918.
- ↑ Wagner, K. R.; Hua, Y.; De Courten-Myers, G. M.; Broderick, J. P.; Nishimura, R. N.; Lu, S. Y.; Dwyer, B. E. (2000). "Tin-mesoporphyrin, a potent hemeoxygenase inhibitor, for treatment of intracerebral hemorrhage: in vivo and in vitro studies". Cellular and Molecular Biology (Noisy-Le-Grand, France) 46 (3): 597–608. PMID 10872746. https://pubmed.ncbi.nlm.nih.gov/10872746/.
This article needs additional or more specific categories. (December 2023) |
 |

