Chemistry:Transhalogenation
Transhalogenation is a substitution reaction in which the halide of a halide compound is exchanged for another halide.[1]
Finkelstein reaction
A common method is halide metathesis. An example is the conversion of alkyl chloride into alkyl fluoride:
- C3H5-Cl + NaF → R-F + NaCl
This kind of reaction is called Finkelstein reaction.[2] However, it is also possible, for example, to produce phosphorus fluoride compounds by transhalogenating chlorine, bromine or iodine bound to phosphorus with a metal fluoride.[3]
Details and biological use
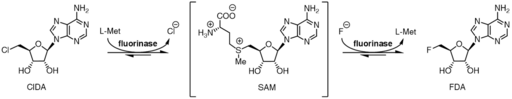
As a halogen source for transhalogenation, metal halides (such as sodium fluoride or lithium fluoride) are often used, but also the use of onium halides is possible.[2] Transhalogenation has been described as a gentle method for the synthesis of fluoroorganylboranes.[4] It is also possible to produce aryliodides from the corresponding aryl chlorides or aryl bromides.[5]
One investigation showed a possibility to perform transhalogenation by means of genetically modified enzymes (haloalkanes dehalogenases, HLDs).[6]
Literature
- Yoel Sasson (2009-12-15). "Formation of Carbon-Halogen Bonds (Cl, Br, I)". PATai's Chemistry of Functional Groups. Chichester, UK: John Wiley & Sons, Ltd. pp. pat0011. doi:10.1002/9780470682531.pat0011. ISBN 978-0-470-68253-1.
- "transhalogenation - Wiktionary". 27 October 2018. https://en.wiktionary.org/wiki/transhalogenation.
References
- ↑ Hudlicky, Milos; Hudlicky, Tomas (1983). "Formation of carbon-halogen bonds". Halides, Pseudo-Halides and Azides: Part 2 (1983). pp. 1021–1172. doi:10.1002/9780470771723.ch3. ISBN 9780470771723.
- ↑ 2.0 2.1 Yoel Sasson (2009-12-15). "Formation of Carbon-Halogen Bonds (Cl, Br, I)". PATai's Chemistry of Functional Groups. Chichester, UK: John Wiley & Sons, Ltd. pp. pat0011. doi:10.1002/9780470682531.pat0011. ISBN 978-0-470-68253-1.
- ↑ "Verfahren zur Transhalogenierung einer Halogenphosphor-Verbindung mit Fluorwasserstoff" DE patent 68918542T, published 1989-08-30
- ↑ Gerd Bir, Wolfgang Schacht, Dieter Kaufmann (1988-02-23), "Eine allgemeine, einfache und schonende Synthesemethode für Fluororganylborane" (in German), Journal of Organometallic Chemistry 340 (3): pp. 267–271, doi:10.1016/0022-328X(88)80020-2, ISSN 0022-328X
- ↑ Alex C. Bissember, Martin G. Banwell (2009-07-03), "Microwave-Assisted Trans-Halogenation Reactions of Various Chloro-, Bromo-, Trifluoromethanesulfonyloxy- and Nonafluorobutanesulfonyloxy-Substituted Quinolines, Isoquinolines, and Pyridines Leading to the Corresponding Iodinated Heterocycles†", The Journal of Organic Chemistry 74 (13): pp. 4893–4895, doi:10.1021/jo9008386, ISSN 0022-3263, PMID 19480440
- ↑ Andy Beier, Jiri Damborsky, Zbynek Prokop (2019-04-17), "Transhalogenation Catalysed by Haloalkane Dehalogenases Engineered to Stop Natural Pathway at Intermediate", Advanced Synthesis & Catalysis: pp. adsc.201900132, doi:10.1002/adsc.201900132, ISSN 1615-4150
 |
