Chemistry:Ugi reaction
| Ugi reaction | |
|---|---|
| Named after | Ivar Karl Ugi |
| Reaction type | Coupling reaction |
| Identifiers | |
| Organic Chemistry Portal | ugi-reaction |
| RSC ontology ID | RXNO:0000129 |
In organic chemistry, the Ugi reaction is a multi-component reaction involving a ketone or aldehyde, an amine, an isocyanide and a carboxylic acid to form a bis-amide.[1][2][3][4] The reaction is named after Ivar Karl Ugi, who first reported this reaction in 1959.
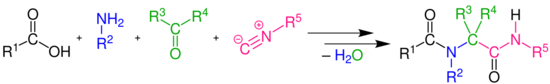
The Ugi reaction is exothermic and usually complete within minutes of adding the isocyanide. High concentration (0.5M - 2.0M) of reactants give the highest yields. Polar, aprotic solvents, like DMF, work well. However, methanol and ethanol have also been used successfully. This uncatalyzed reaction has an inherent high atom economy as only a molecule of water is lost, and the chemical yield in general is high. Several reviews have been published.[5][6][7][8][9][10][11][12][excessive citations]
Due to the reaction products being potential protein mimetics there have been many attempts to development an enantioselective Ugi reaction,[13] the first successful report of which was in 2018.[14]
Reaction mechanism
One plausible reaction mechanism is depicted below:[15]
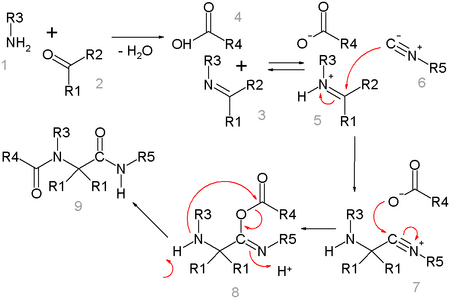
Amine 1 and ketone 2 form the imine 3 with loss of one equivalent of water. Proton exchange with carboxylic acid 4 activates the iminium ion 5 for nucleophilic addition of the isocyanide 6 with its terminal carbon atom to nitrilium ion 7. A second nucleophilic addition takes place at this intermediate with the carboxylic acid anion to 8. The final step is a Mumm rearrangement with transfer of the R4 acyl group from oxygen to nitrogen. All reaction steps are reversible except for the Mumm rearrangement, which drives the whole reaction sequence.
In the related Passerini reaction (lacking the amine) the isocyanide reacts directly with the carbonyl group but other aspects of the reaction are the same. This reaction can take place concurrently with the Ugi reaction, acting as a source of impurities.
Variations
Combination of reaction components
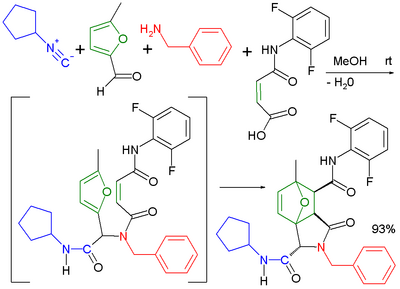
The usage of bifunctional reaction components greatly increases the diversity of possible reaction products. Likewise, several combinations lead to structurally interesting products. The Ugi reaction has been applied in combination with an intramolecular Diels-Alder reaction[16] in an extended multistep reaction.
A reaction in its own right is the Ugi–Smiles reaction with the carboxylic acid component replaced by a phenol. In this reaction the Mumm rearrangement in the final step is replaced by the Smiles rearrangement.[17]
 |
| |
| Ugi–Diels–Alder reaction | Ugi–Smiles reaction |
Another combination (with separate workup of the Ugi intermediate) is one with the Buchwald–Hartwig reaction.[18] In the Ugi–Heck reaction a Heck aryl-aryl coupling takes place in a second step.[19]
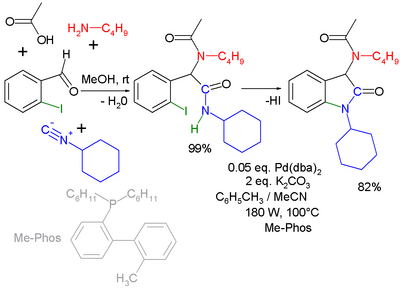 |
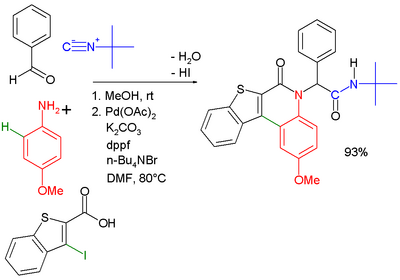
| |
| Ugi–Buchwald–Hartwig reaction [20] | Ugi–Heck reaction [21] |
Combination of amine and carboxylic acid
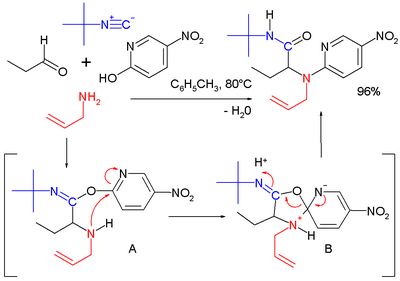
Several groups have used β-amino acids in the Ugi reaction to prepare β-lactams.[22] This approach relies on acyl transfer in the Mumm rearrangement to form the four-membered ring. The reaction proceeds in moderate yield at room temperature in methanol with formaldehyde or a variety of aryl aldehydes. For example, p-nitrobenzaldehyde reacts to form the β-lactam shown in 71% yield as a 4:1 diastereomeric mixture:
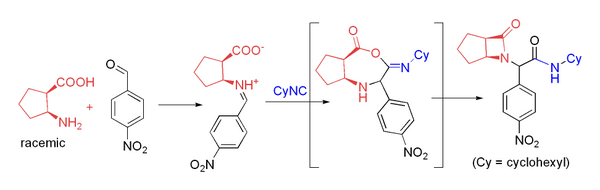
Combination of carbonyl compound and carboxylic acid
Zhang et al.[23] have combined aldehydes with carboxylic acids and used the Ugi reaction to create lactams of various sizes. Short et al.[24] have prepared γ-lactams from keto-acids on solid-support.
Applications
Chemical libraries
The Ugi reaction is one of the first reactions to be exploited explicitly to develop chemical libraries. These chemical libraries are sets of compounds that can be tested repeatedly. Using the principles of combinatorial chemistry, the Ugi reaction offers the possibility to synthesize a great number of compounds in one reaction, by the reaction of various ketones (or aldehydes), amines, isocyanides and carboxylic acids. These libraries can then be tested with enzymes or living organisms to find new active pharmaceutical substances. One drawback is the lack of chemical diversity of the products. Using the Ugi reaction in combination with other reactions enlarges the chemical diversity of possible products. Recently, a breakthrough has been made in the field of covalent organic frameworks (COFs), where Ugi reaction is being utilized to introduce different functional handles into the COFs by post-synthetic modification.[25] With this new promising strategy, it is believed a library of COFs can be prepared with useful functional handles for various important applications.
Examples of Ugi reaction combinations:
- Isoquinolines from Ugi and Heck reactions.[26]
Pharmaceutical industry
Crixivan can be prepared using the Ugi reaction.[27]
Additionally, many of the caine-type anesthetics are synthesized using this reaction. Examples include lidocaine and bupivacaine.
See also
References
- ↑ "Versuche mit Isonitrilen". Angew. Chem. 71 (11): 386. 1959. doi:10.1002/ange.19590711110.
- ↑ "Über ein neues Kondensations-Prinzip". Angew. Chem. 72 (7–8): 267–268. 1960. doi:10.1002/ange.19600720709. Bibcode: 1960AngCh..72..267U.
- ↑ Ugi, I. (1962). "The α-Addition of Immonium Ions and Anions to Isonitriles Accompanied by Secondary Reactions". Angewandte Chemie International Edition in English 1 (1): 8–21. doi:10.1002/anie.196200081.
- ↑ "Ugi Multicomponent Reaction". Org. Synth. 94: 54–65. 2017. doi:10.15227/orgsyn.094.0054. https://www.rug.nl/research/portal/en/publications/ugi-multicomponent-reaction(9c1801e6-dd7f-4c54-a121-4d1a92fe9b2b).html.
- ↑ Tripolitsiotis, Nikolaos P.; Thomaidi, Maria; Neochoritis, Constantinos G. (2020-11-15). "The Ugi Three-Component Reaction; a Valuable Tool in Modern Organic Synthesis". European Journal of Organic Chemistry 2020 (42): 6525–6554. doi:10.1002/ejoc.202001157. ISSN 1434-193X. https://chemistry-europe.onlinelibrary.wiley.com/doi/full/10.1002/ejoc.202001157.
- ↑ "The Passerini and Ugi Reactions". Comprehensive Organic Synthesis. 2. Oxford: Pergamon. 1991. pp. 1083–1109. ISBN 0-08-040593-2.
- ↑ "The Chemistry of Isocyanides, their MultiComponent Reactions and their Libraries". Molecules 8: 53–66. 2003. doi:10.3390/80100053. http://www.mdpi.org/molecules/papers/80100053.pdf.
- ↑ "The Passerini Reaction". Organic Reactions. 65. Wiley. 2005. ISBN 0-471-68260-8.)
- ↑ "Recent advances in heterocycle generation using the efficient Ugi multiple-component condensation reaction". Current Opinion in Drug Discovery & Development 8 (6): 776–88. November 2005. PMID 16312152.
- ↑ "The multicomponent reactions and their libraries for natural and preparative chemistry". Combinatorial Chemistry & High Throughput Screening 4 (1): 1–34. February 2001. doi:10.2174/1386207013331291. PMID 11281825.
- ↑ "Maximizing synthetic efficiency: multi-component transformations lead the way". Chemistry: A European Journal 6 (18): 3321–9. September 2000. doi:10.1002/1521-3765(20000915)6:18<3321::AID-CHEM3321>3.0.CO;2-A. PMID 11039522.
- ↑ "Multicomponent Reactions with Isocyanides". Angewandte Chemie 39 (18): 3168–3210. September 2000. doi:10.1002/1521-3773(20000915)39:18<3168::AID-ANIE3168>3.0.CO;2-U. PMID 11028061.
- ↑ "Still Unconquered: Enantioselective Passerini and Ugi Multicomponent Reactions". Accounts of Chemical Research 51 (5): 1290–1300. May 2018. doi:10.1021/acs.accounts.8b00105. PMID 29708723.
- ↑ "Asymmetric phosphoric acid-catalyzed four-component Ugi reaction". Science 361 (6407): eaas8707. September 2018. doi:10.1126/science.aas8707. PMID 30213886.
- ↑ "Catalytic, enantioselective alpha-additions of isocyanides: Lewis base catalyzed Passerini-type reactions". The Journal of Organic Chemistry 70 (24): 9667–76. November 2005. doi:10.1021/jo050549m. PMID 16292793.
- ↑ "Complexity-enhancing acid-promoted rearrangement of tricyclic products of tandem Ugi 4CC/intramolecular Diels-Alder reaction". The Journal of Organic Chemistry 71 (25): 9544–7. December 2006. doi:10.1021/jo061825f. PMID 17137394.
- ↑ "Direct access to heterocyclic scaffolds by new multicomponent Ugi-Smiles couplings". Organic Letters 8 (18): 4019–21. August 2006. doi:10.1021/ol061605o. PMID 16928063.
- ↑ "Rapid access to oxindoles by the combined use of an Ugi four-component reaction and a microwave-assisted intramolecular Buchwald-Hartwig amidation reaction". Organic Letters 8 (19): 4351–4. September 2006. doi:10.1021/ol061755z. PMID 16956224.
- ↑ "Synthesis of functionalized quinolines via Ugi and Pd-catalyzed intramolecular arylation reactions". Journal of Combinatorial Chemistry 8 (5): 696–704. 2006. doi:10.1021/cc060066b. PMID 16961408.
- ↑ Second part microwave accelerated reaction with Pd(dba)2 and phosphine ligand Me-Phos
- ↑ The Heck step takes place with palladium(II) acetate, dppf ligand potassium carbonate and tetra-n-butylammonium bromide in dimethylformamide
- ↑ "Liquid-phase combinatorial synthesis of alicyclic beta-lactams via Ugi four-component reaction". Organic Letters 4 (11): 1967–9. May 2002. doi:10.1021/ol025986r. PMID 12027659.
- ↑ "Unique Structures Generated by Ugi 3CC Reactions Using Bifunctional Starting Materials Containing Aldehyde and Carboxylic Acid". The Journal of Organic Chemistry 64 (3): 1074–1076. February 1999. doi:10.1021/jo982192a. PMID 11674195.
- ↑ "A solid-phase combinatorial method for the synthesis of novel 5- and 6-membered ring lactams". Tetrahedron Letters 38 (3): 359–362. 1997. doi:10.1016/S0040-4039(96)02303-9.
- ↑ "General Strategy for Incorporation of Functional Group Handles into Covalent Organic Frameworks via the Ugi Reaction". Journal of the American Chemical Society 145 (11): 6230–6239. March 2023. doi:10.1021/jacs.2c12440. PMID 36892967.
- ↑ "Concise synthesis of isoquinoline via the Ugi and Heck reactions". Organic Letters 6 (18): 3155–8. September 2004. doi:10.1021/ol048791n. PMID 15330611.
- ↑ "An efficient asymmetric hydrogenation approach to the synthesis of the Crixivan piperazine intermediate". Tetrahedron Letters 39 (38): 6823–6826. 1998. doi:10.1016/S0040-4039(98)01484-1.
 |
