Chemistry:Upsalite
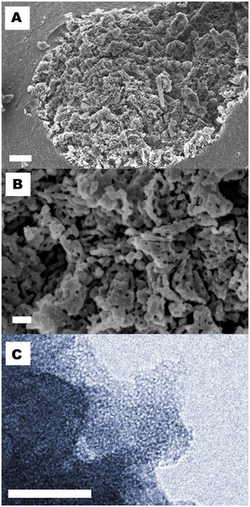
Upsalite is an anhydrous form of magnesium carbonate first reported in July 2013.[1][2] With a surface area of 800 m² per gram, Upsalite is reported to have the highest surface area measured for an alkali earth metal carbonate ever created. It is found to adsorb more water at low relative humidities better than the best materials previously available — the hygroscopic zeolites. Further, upsalite can release that water at lower temperatures than zeolites, requiring less energy.
Naming
The material was given the name Upsalite as a reference to Uppsala University where it was first reported at the lab. There is only one "p" in "upsalite", even though the university's town is spelled "Uppsala". However, "Upsala" is the old spelling of the town, and there is only one "p" in the (Neo-)Latin name of the university, Universitas Regia Upsaliensis.
Synthesis
At the laboratory, magnesium oxide (MgO) and methanol are reacted under a carbon dioxide (CO2) pressure of 3 bar at 50 °C for 3 h.[3] Cooling down results in a gel which after depressurization is solidified in a furnace at 70 °C for 2 days. This material can be calcined at 300 °C, which results in pure dry MgCO3 (Upsalite). The average pore size of Upsalite can be accurately controlled from 2 - 20 nm by adjusting the solidification process.[4]
Potential uses
Due to its capacity to absorb both water and oil and that it can be loaded with chemical substances it has many applications within the cosmetic, sports, consumables and pharmaceutical field. Due to its highly porous property, Upsalite can potentially be used to be used as a drug delivery vehicle, particularly for poorly soluble drugs. Controlled release can be achieved by pore size adjustment or post synthesis modification of the material.[5]It can also be potentially used for collection of toxic waste, chemicals or oil spill and for odor control, sanitation after fire, and the collection of water from any source containing it.[1]
References
- ↑ 1.0 1.1 A Template-Free, Ultra-Adsorbing, High Surface Area Carbonate Nanostructure Forsgren J, Frykstrand S, Grandfield K, Mihranyan A, Strømme M (2013) A Template-Free, Ultra-Adsorbing, High Surface Area Carbonate Nanostructure. PLoS ONE 8(7): e68486. doi:10.1371/journal.pone.0068486
- ↑ "Researchers Develop Record Breaking Magnesium Carbonate Material". SciTech Daily. 2013-07-18. http://scitechdaily.com/researchers-develop-record-breaking-magnesium-carbonate-material/. Retrieved 2013-07-19.
- ↑ Frykstrand, S., Forsgen, J., Mihranyan, A., Stromme, M., On the pore-forming mechanism of Upsalite, a micro- and mesoporous magnesium carbonate, Microporous and Mesoporous Materials 190 (2014) 99-104.
- ↑ Cheung, Ocean; Zhang, Peng; Frykstrand, Sara; Zheng, Haoquan; Yang, Taimin; Sommariva, Marco; Zou, Xiaodong; Strømme, Maria (2016). "Nanostructure and pore size control of template-free synthesised mesoporous magnesium carbonate" (in en). RSC Advances 6 (78): 74241–74249. doi:10.1039/c6ra14171d. ISSN 2046-2069. https://pubs.rsc.org/en/Content/ArticleLanding/2016/RA/C6RA14171D.
- ↑ Vall, Maria; Zhang, Peng; Gao, Ao; Frykstrand, Sara; Cheung, Ocean; Strømme, Maria (May 2017). "Effects of amine modification of mesoporous magnesium carbonate on controlled drug release". International Journal of Pharmaceutics 524 (1-2): 141–147. doi:10.1016/j.ijpharm.2017.03.063. ISSN 0378-5173. https://linkinghub.elsevier.com/retrieve/pii/S0378517317302508.
External links
- Forsgren, Johan; Frykstrand, Sara; Grandfield, Kathryn; Mihranyan, Albert; Strømme, Maria (July 17, 2013). A Template-Free, Ultra-Adsorbing, High Surface Area Carbonate Nanostructure. doi:10.1371/journal.pone.0068486. Bibcode: 2013PLoSO...868486F.