Chemistry:Varrentrapp reaction
The Varrentrapp reaction, also named Varrentrapp degradation, is a name reaction in the organic chemistry. It is named after Franz Varrentrapp, who described this reaction in 1840.[1] The reaction entails the degradation of an unsaturated carboxylic acid into a saturated acid with two fewer carbon atoms and acetic acid. The fragmentation is induced by action of molten alkali.[2]
Stoichiometry and mechanism
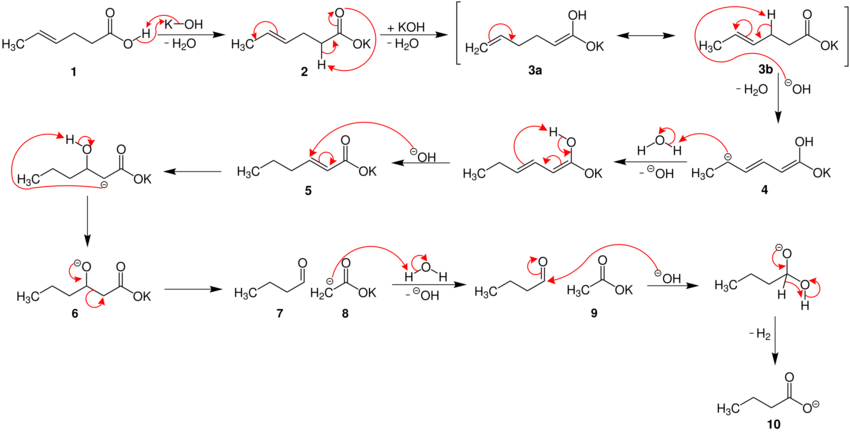
Below, the reaction mechanism is shown for the degradation of (E)-4-hexenoic acid.[3]
First, the carboxylic acid reacts with the caustic potash 1 to form its carboxylate. A series of base-catalyzed isomerizations leads to migration of the alkene into conjugation with the carboxylate (from (2 to 5). Reaction of this α,β-unsaturated carbonyl compound with hydroxide leads to a retro-aldol-condensation reaction, fragmenting the molecule into a shortened carbon chain with an aldehyde (7) and a separate acetate (9). Hydroxide then causes dehydrogenation of the aldehyde to form an acid (10).
Further insight is obtained by study of the Varrentrapp reaction for the conversion of oleic acid to palmitic acid. If the reaction is quenched before formation of the acetate, the recovered C18 acid consists of numerous isomers of octadecenoic acid (but not α,β-octadecenoic acid).[2] This observation suggests that the base (KOH) isomerizes the double bond. It is speculated that this occurs via deprotonation of the allylic C-H's.[4]
Likewise cinnamic acid is converted to benzoic acid.[5]
Applications
The reaction conditions are harsh: medium molten potassium hydroxide at temperatures in the range of 250 to 300 °C. The reaction has been of some importance in structure elucidation of certain fatty acids, but has little practical synthetic value.[6][4] The original 1840 Varrentrapp reaction concerned the conversion of oleate to palmitate and acetate.[1]
References
- ↑ 1.0 1.1 F. Varrentrapp, Ann., 36, 196 (1840)
- ↑ 2.0 2.1 Kwart, Harold; King, Kenneth (1969). "Rearrangement and cyclization reactions of carboxylic acids and esters". in S. Patai. PATAI'S Chemistry of Functional Groups: Carboxylic Acids and Esters (1969). pp. 341–373. doi:10.1002/9780470771099.ch8. ISBN 9780470771099.
- ↑ Wang, Zerong (2009) (in German), Comprehensive Organic Name Reactions and Reagents, New Jersey: John Wiley & Sons, pp. 2864–2868, ISBN 978-0-471-70450-8
- ↑ 4.0 4.1 Bonner, William A.; Rewick, Robert T. (1962). "A Mechanism for the Cleavage of Unsaturated Acids with Molten Alkali". J. Am. Chem. Soc. 84 (12): 2334–2337. doi:10.1021/ja00871a013.
- ↑ Windholz, M. (1976) (in German), The Merck Index, Rahway: Merck & Co, pp. ONR-90, ISBN 0-911910-26-3
- ↑ Organic reactions in strong alkalis-I : Fission of ethylenic acids (the Varrentrapp reaction) Tetrahedron, Volume 8, Issues 3–4, 1960, Pages 221–238 R. G. Ackman, Patrick Linstead, B. J. Wakefield and B. C. L. Weedon doi:10.1016/0040-4020(60)80031-2
 |