Chemistry:Vilsmeier–Haack reaction
| Vilsmeier–Haack reaction | |
|---|---|
| Named after | Anton Vilsmeier Albrecht Haack |
| Reaction type | Substitution reaction |
| Identifiers | |
| Organic Chemistry Portal | vilsmeier-reaction |
| RSC ontology ID | RXNO:0000055 |
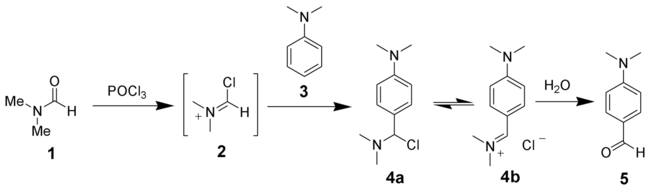
The Vilsmeier–Haack reaction (also called the Vilsmeier reaction) is the chemical reaction of a substituted formamide (1) with phosphorus oxychloride and an electron-rich arene (3) to produce an aryl aldehyde or ketone (5):
- RC(=O)NR′R″ + HArZ + POCl3 + H2O → RC(=O)ArZ + NR′R″H + HCl + H3PO4
The reaction is named after Anton Vilsmeier and Albrecht Haack.[1][2][3]
For example, benzanilide and dimethylaniline react with phosphorus oxychloride to produce an unsymmetrical diaryl ketone.[4] Similarly, anthracene is formylated at the 9-position.[5] The reaction of anthracene with N-methylformanilide, also using phosphorus oxychloride, gives 9-anthracenecarboxaldehyde:
In general, the electron-rich arene (3) must be much more active than benzene for the reaction to proceed; phenols or anilines are good substrates.[6]
Reaction mechanism
The reaction of a substituted amide with phosphorus oxychloride gives a substituted chloroiminium ion (2), also called the Vilsmeier reagent. The initial product is an iminium ion (4b), which is hydrolyzed to the corresponding ketone or aldehyde during workup.[7]
See also
- Formylation reaction
Further reading
- Mallegol, T.; Gmouh, S.; Aït Amer Meziane, M.; Blanchard-Desce, M.; Mongin, O. (2005). "Practical and Efficient Synthesis of Tris(4-formylphenyl)amine, a Key Building Block in Materials Chemistry". Synthesis 2005 (11): 1771–1774. doi:10.1055/s-2005-865336.
- Bélanger, G.; Larouche-Gauthier, R.; Ménard, F.; Nantel, M.; Barabé, F. (2005). "Addition of Tethered Nonaromatic Carbon Nucleophiles to Chemoselectively Activated Amides". Org. Lett. 7 (20): 4431–4. doi:10.1021/ol0516519. PMID 16178551.
- A widely-recommended procedure: doi:10.1055/sos-SD-213-00191
References
- ↑ Vilsmeier, Anton; Haack, Albrecht (1927). "Über die Einwirkung von Halogenphosphor auf Alkyl-formanilide. Eine neue Methode zur Darstellung sekundärer und tertiärer p-Alkylamino-benzaldehyde" (in de). Berichte der Deutschen Chemischen Gesellschaft zu Berlin 60: 119–122. doi:10.1002/cber.19270600118.
- ↑ Meth-Cohn, O.; Stanforth, S. P. (1991). "The Vilsmeier–Haack Reaction (Review)". Compr. Org. Synth. 2: 777–794. doi:10.1016/B978-0-08-052349-1.00049-4.
- ↑ Campaigne, E.; Archer, W. L.. "Formylation of dimethylaniline". Organic Syntheses 33: 27. doi:10.15227/orgsyn.033.0027. http://www.orgsyn.org/demo.aspx?prep=cv4p0331.; Collective Volume, 4, pp. 331
- ↑ Hurd, C. D.; Webb, C. N. (1927). "Vilsmeyer–Haack reaction of benzanilide and dimethylaniline". Organic Syntheses 7: 24. doi:10.15227/orgsyn.007.0024.
- ↑ Fieser, F. L.; Hartwell, J. L.; Jones, J. E.; Wood, J. H.; Bost, R. W. (1940). "Formylation of anthracene". Organic Syntheses 20: 11. doi:10.15227/orgsyn.020.0011.
- ↑ Smith, Michael B. (2020). March's Organic Chemistry (8th ed.). Wiley. p. 664.
- ↑ Jones, G.; Stanforth, S. P. (2000). "The Vilsmeier Reaction of Non-Aromatic Compounds". Org. React. 56 (2): 355–686. doi:10.1002/0471264180.or056.02.
 |