DICONDE
Digital Imaging and Communication for Nondestructive Evaluation (DICONDE) is a vendor-neutral digital data storage and transmission protocol that defines the organization of nondestructive testing (NDT) inspection data and associated metadata in a standard format. DICONDE is based on and inherits from the universally adopted medical standard, DICOM, which facilitates the interoperability of imaging, video, and signal data acquisition equipment through data storage, query, and network communication protocols.
The ASTM International standards organization maintains and holds the copyright to the relevant DICONDE published standards, including a tutorial guide designated as E3169[1]. Development and maintenance of the standard is handled by committee E07[2] on nondestructive testing. Subcommittee E07.11 on DICONDE is concerned with the formulation of standards for the communication and storage of data generated by all nondestructive testing methodologies capable of handling data in an electronic format.
ASTM maintains a page dedicated to DICONDE and openly provides resources on the ASTM DICONDE home page[3].
History
DICONDE is a standard developed by ASTM. In the late 1990s, most vendor NDT acquisition systems were saving image data and their corresponding metadata in proprietary formats. This made it difficult for customers to extract, analyze, and use the image data using software outside of the proprietary vendor systems. A need in the industry was raised for a standard data format that would facilitate interoperability between software applications. With DICOM gaining a universal level of acceptance over the past decades in the medical industry[4], a derivation was proposed for the manufacturing inspection industry’s acquisition manufacturers to share image data.
DICONDE was initially publicly announced in October 2003 as part of a Standardization News article[5] and then ratified in 2004 as ASTM standard E2339[6]. DICONDE was created as a superset to DICOM on which it is based. Being a superset, DICONDE inherits all the changes that progress in the DICOM standard which allows for continuous improvement and expansion of the standard.
The main E2339 standard was adapted to form DICONDE 2.0 in 2006. As part of that effort, specialized NDE data modules such as the indication module and the NDE approval module were added. Additional modality-specific, or class-specific, standards that inherit from E2339 were developed, including one for ultrasonic testing (E2663 in 2008), computed radiography (E2738 in 2009), digital radiography (E2699 in 2010), computed tomography (E2767 in 2011), and an additional information object for eddy current, which is a specialized class developed specifically for DICONDE (E2934 in 2013).
The interoperability standard E3147 was added in 2018 to define the extent to which two or more NDT DICONDE-compliant applications, or application entities, communicate, interconnect, or interoperate together and to provide a means for verification and validation between vendor acquisition systems. Later that year, the DICONDE guide E3169 was introduced for system purchasers and system manufacturers getting started with supporting DICONDE[7][8].
Applications
DICONDE is used worldwide to store, exchange, and transmit NDT inspection data. Unlike in the medical field, adoption of DICONDE has been slower because of a lack of regulatory requirements for interoperability. With an increasing importance of Industry 4.0, however, DICONDE offers excellent functionality for networking inspection equipment and for design of paperless, truly digital testing workflows.
DICONDE and DICOM
Many of the information modules and attributes defined within the DICOM standard can be adapted to DICONDE usage either directly or by close analogy. For example, the Image Pixel Information Module or the Device Serial Number Attribute are the same for medical or manufacturing usage and used directly by DICONDE. Although there is no concept of a patient in industrial inspection applications, the component being inspected is partially analogous to the patient in medical imaging applications. In DICONDE the Patient Information Module is transformed into the Component Information Module and the Patient ID Attribute is reused as Component ID. Examples:
- DICOM Patient ID becomes DICONDE Component ID
- DICOM Patient Name becomes DICONDE Component Name
- DICOM Patient Birth Date becomes DICONDE Component Manufacturing Date
Some DICOM information modules or attributes, however, have no correlation to nondestructive evaluation. In those cases, the user optional portions are typically ignored by DICONDE allowing all DICONDE compliant objects to be DICOM compliant. Some concepts, like the eddy current modality are used in the NDE arena but are not used in medicine and therefore are not available in the DICOM constructs. In these cases, DICONDE introduces new information modules or attributes as needed to describe the required metadata, in cooperation with the DICOM working group.
The DICONDE standard has introduced the NDE Geometry Module to contain data about the part and scanner/detector coordinate systems required by some inspection techniques. In similar fashion, a new NDE Indication Module was adopted to facilitate the storage of indications identified by operators in image data. Another major difference between the two standards is the increased use of code sequences within the medical industry. In NDE, wider ranges of objects are inspected and sets of predefined codes typically are not used. Both raw and calibration data are typically not stored for medical applications where they are required for industrial imaging. Lastly, high-energy sources are not well represented in DICOM.
Inheritance of DICONDE Standards
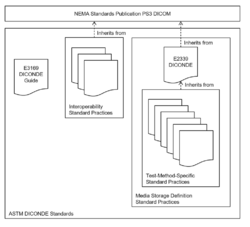
ASTM has developed a standard guide E3169 to act as an entry point to the set of NDE method and interoperability standards it maintains. The standard provides an overview of the relationships of these standards to each other and their relationship to DICOM. DICONDE uses the inheritance concept to simplify the documentation of the standards, effectively inheriting from DICOM except when overriding the medical-specific aspects to relate more closely to the manufacturing industry. All DICONDE-compliant objects are specified to be DICOM-compliant.
Active Standards
Active Method Standards
E2738 - Standard Practice for Digital Imaging and Communication in Nondestructive Evaluation (DICONDE) for Computed Radiography (CR) Test Methods
E2699 - Standard Practice for Digital Imaging and Communication in Nondestructive Evaluation (DICONDE) for Digital Radiographic (DR) Test Methods
E2767 - Standard Practice for Digital Imaging and Communication in Nondestructive Evaluation (DICONDE) for X-ray Computed Tomography (CT) Test Methods
E2663 - Standard Practice for Digital Imaging and Communication in Nondestructive Evaluation (DICONDE) for Ultrasonic Test (UT) Methods
E2934 - Standard Practice for Digital Imaging and Communication in Nondestructive Evaluation (DICONDE) for Eddy Current (EC) Test Methods
Active Related Standards
E1316 - Standard Terminology for Nondestructive Examinations
E2339 - Standard Practice for Digital Imaging and Communication in Nondestructive Evaluation (DICONDE)
E3147 - Standard Practice for Evaluating DICONDE Interoperability of Nondestructive Testing and Inspection Systems
E3169 - Standard Guide for Digital Imaging and Communication in Nondestructive Evaluation (DICONDE)
Connectathons
Similar to work done in the early days of DICOM in the medical industry, ASTM International hosts connectathon events at the E07 bi-annual, meetings allowing vendors and standards developers to work together and cooperatively test their software in an open and collaborative manner[9]. Results of these meetings are documented in the ASTM E07.11 DICONDE task group minutes.
References
- ↑ "ASTM Standard E3169". https://www.astm.org/Standards/E3169.htm.
- ↑ "ASTM Standardization News - Ensuring Infrastructure Integrity". https://sn.astm.org/?q=features/ensuring-infrastructure-integrity-mj13.html.
- ↑ "ASTM DICONDE Homepage". https://www.astm.org/COMMIT/DICONDE_Information.htm.
- ↑ DICOM
- ↑ "ASTM DICONDE Standard - ASTM Standardization News - October 2003". https://www.astm.org/SNEWS/OCTOBER_2003/voelker_oct03.html.
- ↑ "ASTM Standard E2339". https://www.astm.org/Standards/E2339.htm.
- ↑ "ASTM Standardization News - Back to a Digital Future". https://sn.astm.org/?q=features/back-digital-future-ja11.html.
- ↑ "Quality Magazine - DICONDE in Digital NDT Taking Hold". https://www.qualitymag.com/articles/94832-diconde-in-digital-ndt-taking-hold.
- ↑ "ASTM Standardization News - Nondestructive Testing Committee Supports Data Interoperability with New Standard, “Connect-a-Thon” Events". https://sn.astm.org/?q=update/nondestructive-testing-committee-supports-data-interoperability-new-standard-“connect-thon”.
