Earth:Brine rejection
Brine rejection is a process that occurs when salty water freezes. The salts do not fit in the crystal structure of water ice, so the salt is expelled.
Since the oceans are salty, this process is important in nature. Salt rejected by the forming sea ice drains into the surrounding seawater, creating saltier, denser brine. The denser brine sinks, influencing ocean circulation.
Formation
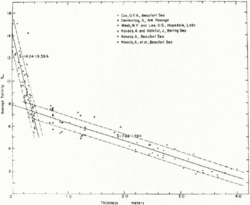
As water reaches the temperature where it begins to crystallize and form ice, salt ions are rejected from the lattices within the ice and either forced out into the surrounding water, or trapped among the ice crystals in pockets called brine cells. Generally, sea ice has a salinity ranging from 0 psu at the surface to 4 psu at the base.[1] The faster that this freezing process occurs, the more brine cells are left in the ice. Once the ice reaches a critical thickness, roughly 15 cm, the concentration of salt ions in the liquid around the ice begins to increase, as leftover brine is rejected from the cells.[1] This increase is associated with the appearance of strong convective plumes, which flow from channels and within the ice and carry a significant salt flux. The brine that drains from the newly formed ice is replaced by a weak flow of relatively fresh water, from the liquid region below it. The new water partially freezes within the pores of the ice, increasing the solidity of the ice.
As sea ice ages and thickens, the initial salinity of the ice decreases due to the rejection of brine over time [Fig. 2].[1] While the sea ice ages, desalinization occurs to such a degree that some multiyear ice has a salinity of less than 1 PSU.[2] This occurs in three different ways:
- solute diffusion - this depends on the fact that brine inclusions trapped in ice will begin to migrate toward the warmer end of the ice block. The ice block is warmest at the water-ice interface, thus pushing the brine out into the water surrounding the ice.[3]
- gravity drainage - Gravity drainage involves the movement of brine due to differences in density between brine in the interior of the ice and brine in the seawater outside of the ice, which occurs due to the development of a buoyancy driven convection system.[4]
- expulsion - the migration of brine due to cracking produced by thermal expansion of the ice, or pressure caused by the increased volume of the newly formed ice.[3]
Role in deep water formation and the thermohaline circulation
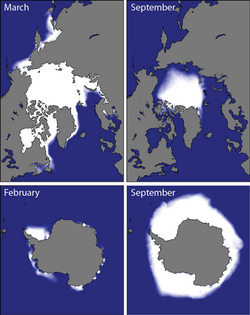
Brine rejection occurs in the sea ice packs around at the north and south poles of the Earth [Fig. 3][clarification needed]. The Arctic Ocean has historically ranged from roughly 14-16 million square kilometers in late winter to roughly 7 million square kilometers each September.[6] The annual increase of ice plays a major role in the movement of ocean circulation and deep water formation. The density of the water below the newly formed ice increases due to the brine rejection. Saltier water can also become colder without freezing.
The dense waters that form in the Arctic are called North Atlantic Deep Waters (NADW), while the Antarctic Bottom Water (AABW) forms in the southern hemisphere. These two areas of brine rejection play an important role in the thermohaline circulation of all of Earth's oceans.
Brinicles
As sea ice freezes, it rejects increasingly salty water, which drains through narrow brine channels that thread through the ice. The brine flowing through the brine channels and out of the bottom of the ice is very cold and salty, so it sinks in the warmer, fresher seawater under the ice, forming a plume. The plume is colder than the freezing point of sea water under the ice, so the seawater can freeze where it touches the plume. Ice freezing around the edges of the plume gradually builds a hollow icicle-like tube, called a brinicle. These frozen stalactite-like forms are fragile during early stages, but if brine drainage ceases, they may freeze solid. In calm waters, brinicles can reach the sea floor, freezing it fairly abruptly.[7]
Climate change
The deep ocean basins are stably stratified, so mixing of surface waters with the deep ocean waters occurs only very slowly. The dissolved CO2 of the surface waters of the ocean is roughly in equilibrium with the partial pressure of CO2 in the atmosphere. As atmospheric CO2 levels are rising, the oceans are absorbing some CO2 from the atmosphere. When surface waters sink, they carry considerable amounts of CO2 into the deep oceans, away from the atmosphere. Because these waters are able to contain a large amount of CO2, they have helped slow the rise in atmospheric CO2 concentrations, thus slowing some aspects of climate change.
Climate change could have different effects on ice melt and brine rejection. Previous studies have suggested that as ice cover thins, it will become a weaker insulator, resulting in larger ice production during the autumn and winter.[8] The consequent increase in winter brine rejection will drive ocean ventilation, and strengthen the inflow of warm Atlantic waters. Studies of the last glacial maximum (LGM) have indicated that a drastic reduction in the production of sea ice and thus reduction of brine rejection, would result in the weakening of the stratification in the global deep oceans and in CO2 release into the shallow oceans and the atmosphere, triggering global deglaciation.[9]
Life in channels and surrounding waters
Life in sea ice is energetically demanding, and sets limits at any hierarchical organizational, and organismic level, ranging from molecules to everything that an organism does.[clarification needed][9] Despite this fact, the brine-containing interstices and pockets found in sea ice host a variety of organisms, including bacteria, autotrophic and heterotrophic protists, microalgae, and metazoa.[10]
Brine rejection and the North Pacific Intermediate Water
Brine rejection plays a crucial role in ocean circulation. In coastal polynyas, it is the key to the ventilation of multiple water masses across both the Arctic and Antarctic. A coastal polynya is an area of open water surrounded by ice.[11] The reason coastal polynyas are the most active areas of brine rejection is that these waters often see offshore winds which give the water direct contact with cold air.[12] This leads to heat loss and ice production. One area that is commonly studied to look at these impacts is the coastal polynyas of the Okhotsk Sea. The Okhotsk Sea has wide, shallow shelves, severe wintertime conditions, high background salinity, and easy summertime access making it an ideal study location.[12] There have been many studies done that look at the influence of brine rejection in the Okhotsk Sea.
In a paper done by Shcherbina et al. (2003), they analyze the influence of brine rejection well.[13] Within the Okhotsk Sea, circulation is driven by the brine rejection that occurs in the winter months. As is typical for the rejection of brine, sea ice develops that is 70-90% fresher than seawater. The water underneath becomes saltier and colder, leading to an increase in density. This parcel of water in the Okhotsk Sea is referred to as dense shelf water (DSW). The saltier and colder a water parcel is, the denser it becomes, causing it to sink below other parcels of water. For this reason, the DSW will begin to sink within the water column. The parcel then moves southward along the coast of Sakhalin. From here, the water moves over into the Pacific and ventilates the North Pacific Intermediate Water (NPIW). The NPIW is known to be the densest water in the North Pacific, and it is a key water mass in ocean circulation.
Brine rejection has been shown to ventilate the North Pacific Ocean to a depth of 300-1,000 meters deep. Some studies have even shown it reaching mixing depths of 2,000 meters.[14] The mixing and ventilation of the water column is key in helping to replenish the oxygen within intermediate waters. It can also lead to an upwelling of nutrients which can influence productivity. An increase in primary production can lead to an increase in other organisms such as krill to whales.
References
- ↑ 1.0 1.1 1.2 1.3 Cox, G. F. N.; Weeks, W. F. (1974-01-01). "Salinity Variations in Sea Ice". Journal of Glaciology 13 (67): 109–120. doi:10.1017/S0022143000023418. ISSN 0022-1430. Bibcode: 1974JGlac..13..109C.
- ↑ Talley, L.D.; Pickard, G.L.; Emery, W.J.; Swift, J.H. (2011). Descriptive Physical Oceanography (6 ed.). Elsevier. doi:10.1016/C2009-0-24322-4. ISBN 9780750645522.
- ↑ 3.0 3.1 Lake, R.A.; Lewis, E.L. (1970). "Salt rejection by sea ice during growth". J. Geophys. Res. 75 (3): 583–597. doi:10.1029/jc075i003p00583. Bibcode: 1970JGR....75..583L.
- ↑ Wettlaufer, J. S.; Worster, M. Grae; Huppert, Herbert E. (1997). "Natural convection during solidification of an alloy from above with application to the evolution of sea ice". Journal of Fluid Mechanics 344 (1): 291–316. doi:10.1017/S0022112097006022. Bibcode: 1997JFM...344..291W. https://doi.org/10.1017/S0022112097006022.
- ↑ "Arctic vs. Antarctic | National Snow and Ice Data Center". https://nsidc.org/cryosphere/seaice/characteristics/difference.html.
- ↑ "All About Sea Ice | National Snow and Ice Data Center". https://nsidc.org/cryosphere/seaice/index.html.
- ↑ Davies, Ella (2011-11-23). "'Brinicle' ice finger of death filmed in Antarctic". https://www.bbc.co.uk/nature/15835017.
- ↑ Holland, Marika M.; Bitz, Cecilia M.; Tremblay, Bruno (2006). "Future abrupt reductions in the summer Arctic sea ice". Geophysical Research Letters 33 (23). doi:10.1029/2006GL028024. Bibcode: 2006GeoRL..3323503H. https://doi.org/10.1029/2006GL028024.
- ↑ 9.0 9.1 Thatje, S.; Hillenbrand, C.D.; Mackensen, A.; Larter, R. (2008). "Life hung by a thread: endurance of Antarctic fauna in glacial periods". Ecology 89 (3): 682–692. doi:10.1890/07-0498.1. PMID 18459332. https://eprints.soton.ac.uk/50811/1/Thatje_Ecology_08.pdf.
- ↑ Giannelli, Virginia; Thomas, David N.; Haas, Christian; Kattner, Gerhard; Kennedy, Hilary; Dieckmann, Gerhard S. (2001). "Behaviour of dissolved organic matter and inorganic nutrients during experimental sea-ice formation". Annals of Glaciology 33: 317–321. doi:10.3189/172756401781818572. Bibcode: 2001AnGla..33..317G.
- ↑ Fukamachi, Yasushi; Shirasawa, Kunio; Polomoshnov, Anatoliy M.; Ohshima, Kay I.; Kalinin, Ervin; Nihashi, Sohey; Melling, Humfrey; Mizuta, Genta et al. (2009). "Direct observations of sea-ice thickness and brine rejection off Sakhalin in the Sea of Okhotsk". Continental Shelf Research 29 (11–12): 1541–1548. doi:10.1016/j.csr.2009.04.005. Bibcode: 2009CSR....29.1541F. https://doi.org/10.1016/j.csr.2009.04.005.
- ↑ 12.0 12.1 Shcherbina, Andrey Y.; Talley, Lynne D.; Rudnick, Daniel L. (2004). "Dense water formation on the northwestern shelf of the Okhotsk Sea: 1. Direct observations of brine rejection". Journal of Geophysical Research 109 (C9). doi:10.1029/2003jc002196. Bibcode: 2004JGRC..109.9S08S.
- ↑ Shcherbina, Andrey Y.; Talley, Lynne D.; Rudnick, Daniel L. (2003). "Direct Observations of North Pacific Ventilation: Brine Rejection in the Okhotsk Sea". Science 302 (5652): 1952–1955. doi:10.1126/science.1088692. PMID 14671300. Bibcode: 2003Sci...302.1952S. https://doi.org/10.1126/science.1088692.
- ↑ Detlef, Henrieka; Sosdian, Sindia M.; Belt, Simon T.; Smik, Lukas; Lear, Caroline H.; Kender, Sev; Pearce, Christof; Hall, Ian R. (2020). "Late quaternary sea-ice and sedimentary redox conditions in the eastern Bering Sea – Implications for ventilation of the mid-depth North Pacific and an Atlantic-Pacific seesaw mechanism". Quaternary Science Reviews 248: 106549. doi:10.1016/j.quascirev.2020.106549. Bibcode: 2020QSRv..24806549D. https://doi.org/10.1016/j.quascirev.2020.106549.
External links
- http://www.bbc.com/earth/story/20161219-brinicle-finger-of-death Brinicle Video by BBC
 |
