Earth:Verneuil process
The Verneuil process, also called flame fusion, was the first commercially successful method of manufacturing synthetic gemstones, developed in the late 1800s [1] by the French chemist Auguste Verneuil. It is primarily used to produce the ruby, sapphire and padparadscha varieties of corundum, as well as the diamond simulants rutile and strontium titanate. The principle of the process involves melting a finely powdered substance using an oxyhydrogen flame, and crystallising the melted droplets into a boule. The process is considered to be the founding step of modern industrial crystal growth technology, and remains in wide use to this day.
History
Since the study of alchemy began, there have been attempts to synthetically produce precious stones, and ruby, being one of the prized cardinal gems, has long been a prime candidate.
The first fusion of ruby was achieved by Antoine Lavoisier in 1782, by melting three small rubies together under an oxygen blowpipe.[2] The first ruby to be fused from its chemical constituents was produced by Marc Gaudin in 1834 - though his stones were not transparent.[3] Jacques-Joseph Ébelmen was the first to synthesize transparent rubies in 1847.[4] Ébelmen's rubies were crystallised from a solution rather than through flame fusion.[4] But whilst Ébelmen's rubies were transparent, they were also microscopic.[4] Edmond Frémy would later improve the crystallisation of ruby from a solution to grow larger crystals: first alongside the industrial glass-maker Charles Feil, and latterly alongside his student Auguste Verneuil.[5] By this point Verneuil was already developing the process of flame fusion that would later bear his name.
Verneuil's work on the flame fusion of ruby became apparent in 1886 following the appearance of "Geneva Rubies".[6] These were the first synthetic rubies to be both large and transparent, and the first to be produced on a commercial basis.[6] However, the identity of their inventor, as well as the raw materials used in their initial production, remain matters of debate.[6] Shortly after the appearance of Geneva Rubies, Verneuil and his colleague Auguste Terreil were consulted on their manufacture and were immediately able to demonstrate the production of a flame fusion ruby.[6] By 1892 Verneuil had perfected a more efficient method of flame fusion. In his 'Verneuil furnace' finely ground particles of alumina and chromium oxide were melted by an oxyhydrogen flame of at least 2,000 °C (3,630 °F), and crystallised on a mobile support below the flame, creating a large crystal. Details of this new method were not published by Verneuil until 1902.
By 1910, Verneuil's laboratory had expanded into a 30-furnace production facility, with annual gemstone production by the Verneuil process having reached 1,000 kg (2,200 lb) in 1907. By 1912, production reached 3,200 kg (7,100 lb), and would go on to reach 200,000 kg (440,000 lb) in 1980 and 250,000 kg (550,000 lb) in 2000, led by Hrand Djevahirdjian's factory in Monthey, Switzerland , founded in 1914. The most notable improvements in the process were made in 1932, by S. K. Popov, who helped establish the capability for producing high-quality sapphires in the Soviet Union through the next 20 years. A large production capability was also established in the United States during World War II, when European sources were not available, and jewels were in high demand for their military applications.
The process was designed primarily for the synthesis of rubies, which became the first gemstone to be produced on an industrial scale. However, the Verneuil process could also be used for the production of other stones, including blue sapphire, which required oxides of iron and titanium to be used in place of chromium oxide, as well as more elaborate ones, such as star sapphires, where titania (titanium dioxide) was added and the boule was kept in the heat longer, allowing needles of rutile to crystallise within it. In 1947, the Linde Air Products division of Union Carbide pioneered the use of the Verneuil process for creating such star sapphires, until production was discontinued in 1974 due to overseas competition.
Despite some improvements in the method, the Verneuil process remains virtually unchanged to this day, while maintaining a leading position in the manufacture of synthetic corundum and spinel gemstones. Its most significant setback came in 1917, when Jan Czochralski introduced the Czochralski process, which has found numerous applications in the semiconductor industry, where a much higher quality of crystals is required than the Verneuil process can produce. Other alternatives to the process emerged in 1957, when Bell Labs introduced the hydrothermal process, and in 1958, when Carroll Chatham introduced the flux method. In 1989 Larry P Kelley of ICT, Inc. also developed a variant of the Czochralski process where natural ruby is used as the 'feed' material.
Process
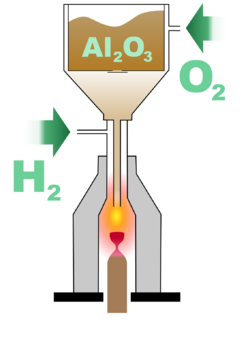

One of the most crucial factors in successfully crystallising an artificial gemstone is obtaining highly pure starting material, with at least 99.9995% purity. In the case of manufacturing rubies, sapphires or padparadscha, this material is alumina. The presence of sodium impurities is especially undesirable, as it makes the crystal opaque. Depending on the desired colouration of the crystal, small quantities of various oxides are added, such as chromium oxide for a red ruby, or ferric oxide and titania for a blue sapphire. Other starting materials include titania for producing rutile, or titanyl double oxalate for producing strontium titanate. Alternatively, small, valueless crystals of the desired product can be used.
This starting material is finely powdered, and placed in a container within a Verneuil furnace, with an opening at the bottom through which the powder can escape when the container is vibrated. While the powder is being released, oxygen is supplied into the furnace, and travels with the powder down a narrow tube. This tube is located within a larger tube, into which hydrogen is supplied. At the point where the narrow tube opens into the larger one, combustion occurs, with a flame of at least 2,000 °C (3,630 °F) at its core. As the powder passes through the flame, it melts into small droplets, which fall onto an earthen support rod placed below. The droplets gradually form a sinter cone on the rod, the tip of which is close enough to the core to remain liquid. It is at that tip that the seed crystal eventually forms. As more droplets fall onto the tip, a single crystal, called a boule, starts to form, and the support is slowly moved downward, allowing the base of the boule to crystallise, while its cap always remains liquid. The boule is formed in the shape of a tapered cylinder, with a diameter broadening away from the base and eventually remaining more or less constant. With a constant supply of powder and withdrawal of the support, very long cylindrical boules can be obtained. Once removed from the furnace and allowed to cool, the boule is split along its vertical axis to relieve internal pressure, otherwise the crystal will be prone to fracture when the stalk is broken due to a vertical parting plane.
When initially outlining the process, Verneuil specified a number of conditions crucial for good results. These include: a flame temperature that is not higher than necessary for fusion; always keeping the melted product in the same part of the oxyhydrogen flame; and reducing the point of contact between the melted product and support to as small an area as possible. The average commercially produced boule using the process is 13 mm (0.51 in) in diameter and 25 to 50 mm (0.98 to 1.97 in) long, weighing about 125 carats (25.0 g). The process can also be performed with a custom-oriented seed crystal to achieve a specific desired crystallographic orientation.

Crystals produced by the Verneuil process are chemically and physically equivalent to their naturally occurring counterparts, and strong magnification is usually required to distinguish between the two. One of the telltale characteristics of a Verneuil crystal is curved growth lines (curved striae) formed as the cylindrical boule grows upwards in an environment with a high thermal gradient; the equivalent lines in natural crystals are straight. Another distinguishing feature is the common presence of microscopic gas bubbles formed due to an excess of oxygen in the furnace; imperfections in natural crystals are usually solid impurities.
See also
- Bridgman–Stockbarger technique
- Czochralski process
- Float-zone silicon
- Kyropoulos process
- Laser-heated pedestal growth
- Micro-pulling-down
- Shelby Gem Factory
References
- ↑ "The Chemical News and Journal of Physical Science". 1891. https://books.google.com/?id=VBpLAAAAYAAJ&pg=PA96&dq=ruby+verneuil#v=onepage&q=ruby%20verneuil&f=false.
- ↑ Evans, James (2020). The History of Synthetic Ruby. Lustre Gemmology. pp. 3-8. ISBN 1916165206.
- ↑ Evans, James (2020). The History of Synthetic Ruby. Lustre Gemmology. pp. 13-15. ISBN 1916165206.
- ↑ 4.0 4.1 4.2 Evans, James (2020). The History of Synthetic Ruby. Lustre Gemmology. pp. 16. ISBN 1916165206.
- ↑ Evans, James (2020). The History of Synthetic Ruby. Lustre Gemmology. pp. 17-27. ISBN 1916165206.
- ↑ 6.0 6.1 6.2 6.3 Evans, James (2020). The History of Synthetic Ruby. Lustre Gemmology. pp. 28-36. ISBN 1916165206.
- Nassau, K. (October 1969). "'Reconstructed' or 'Geneva' ruby". Journal of Crystal Growth 5 (5): 338–344. doi:10.1016/0022-0248(69)90035-9.
- Harris, D. C. (September 2003). "A peek into the history of sapphire crystal growth". Proceedings of SPIE. Window and Dome Technologies VIII 5078: 1–11. doi:10.1117/12.501428. https://zenodo.org/record/1235600.
- Levin, I. H. (June 1913). "Synthesis of precious stones". The Journal of Industrial and Engineering Chemistry 5 (6): 495–500. doi:10.1021/ie50054a022.
- Scheel, H. J. (April 2000). "Historical aspects of crystal growth technology". Journal of Crystal Growth 211 (1–4): 1–12. doi:10.1016/S0022-0248(99)00780-0.
- Imel, D. (May 2005). "What is the procedure by which synthetic rubies are produced?". The Rock Collector 105 (5): 6–8. Archived from the original on October 25, 2005. https://web.archive.org/web/20051025160459/http://www.rochesterlapidary.org/RockCollector/2005/May-2005.pdf.
- R. T. Liddicoat Jr., Gem, McGraw-Hill AccessScience, January 2002, Page 2.
- Hughes, R. W.; Koivula, J. I. (October 2005). "Dangerous Curves: A Reexamination of Verneuil Synthetic Corundum". http://ruby-sapphire.com/verneuil-synthetic-corundum-dangerous-curves.htm.
External links

