Medicine:Activated protein C resistance test
| Activated protein C resistance test | |
|---|---|
| Medical diagnostics | |
| Synonyms | APC resistance test; Activated protein C resistance assay; APC resistance assay; APCR test; APCR assay |
| Test of | Activated protein C resistance, coagulation, hypercoagulability |
The activated protein C resistance (APCR) test is a coagulation test used in the evaluation and diagnosis of activated protein C (APC) resistance, a form of hypercoagulability.[1][2] Hereditary APC resistance is usually caused by the factor V Leiden mutation, whereas acquired APC resistance has been linked to antiphospholipid antibodies, pregnancy, and estrogen therapy.[3][4][5][6] APC resistance can be measured using either an activated partial thromboplastin time (aPTT)-based test or an endogenous thrombin potential (ETP)-based test.[5][4][2]
Methodology
The aPTT-based APC resistance test involves a modified aPTT test performed in the presence and absence of activated protein C (APC).[1][5] The ratio of these aPTT values is calculated and is called the APC sensitivity ratio (APCsr) or simply APC ratio (APCr).[1][5] This ratio is inversely related to the degree of APC resistance.[7] The ETP-based APC resistance test involves the addition of APC to a thrombin generation assay (TGA).[5] This results in an inhibition of thrombin generation as measured by reduction of the endogenous thrombin potential (ETP; area under the thrombin generation curve).[5] The result is expressed as a normalized APC sensitivity ratio (nAPCsr), which corresponds to the ratio of the ETP measured in the presence and absence of APC divided by the same ratio in reference plasma.[5] nAPCsr values range from 0 to 10.[5] Opposite to the case of the APCsr with the aPTT-based APC resistance test, higher nAPCsr values indicate greater APC resistance.[5][8] This is the result of the fact that APC prolongs the aPTT but inhibits thrombin generation.[8]
Whereas the aPTT-based APC resistance test only measures the initiation phase of coagulation, the ETP-based test is a global assay and measures the initiation, propagation, and termination phases of coagulation.[5][9] The initiation phase accounts for less than 5% of total thrombin generation, making aPTT-based tests poorly indicative of hypercoagulability in general.[10][11] The aPTT-based assay is more sensitive to levels of prothrombin and factor VIII, whereas the ETP-based test is more sensitive to levels of tissue factor pathway inhibitor (TFPI) and protein S.[5] The ETP-based test has traditionally been performed using methods such as the calibrated automated thrombogram (CAT) and has been limitedly available due to its technical difficulty.[2] Recently however, a fully automated commercial test system called the ST Genesia has been introduced, and it has been said that this should allow for adoption of TGAs and ETP-based APC resistance tests in routine clinical settings.[2][12]
Influences
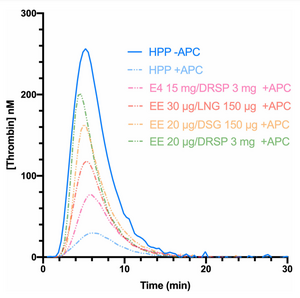
Estrogens are well known to increase APC resistance, which has been described as acquired APC resistance.[2][5][4][13][14] However, the aPTT-based APC resistance test is much less sensitive to the procoagulatory effects of estrogens than is the ETP-based test.[13][14][5][4][2][15] Pregnancy[7] and ethinylestradiol (EE)-containing combined birth control pills increase APC resistance as measured by either the aPTT- or ETP-based test.[4][5][15] EE-containing birth control pills show different degrees of influence on the ETP-based test depending on the progestin, which may be due to varying degrees of androgenic antagonism of ethinylestradiol-mediated procoagulation.[5][4] In contrast to EE-containing birth control pills, studies have not found increased APC resistance with menopausal hormone therapy or with estetrol- or estradiol-containing birth control pills using the aPTT-based test, though increased APC resistance has been shown with the ETP-based test.[14] The increase in APC resistance is much greater with oral estrogens than with transdermal estradiol.[14] Increased APC resistance with both the aPTT-based and ETP-based tests has been observed with feminizing hormone therapy in transgender women, which involves higher doses of estradiol than are used in other contexts.[16][17] EE produces a much stronger increase in APC resistance than does estradiol.[18][17] In relation to this, ethinylestradiol is associated with a higher risk of venous thromboembolism (VTE) than is estradiol.[18][19][20]
History
The aPTT-based APC resistance test was developed in 1993, while the ETP-based test was developed in 1997.[5] For many years, the ETP-based APC resistance test suffered from a lack of standardization which hampered study-to-study comparison.[21] By 2020 however, a validated methodology was developed aiming to propose a standardized and harmonized scale for ETP-based APC resistance, the normalized activated protein C sensitivity ratio (nAPCsr).[21]
References
- ↑ 1.0 1.1 1.2 "Laboratory assessment of Activated Protein C Resistance/Factor V-Leiden and performance characteristics of a new quantitative assay". Transfus Apher Sci 56 (6): 906–913. December 2017. doi:10.1016/j.transci.2017.11.021. PMID 29162399.
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 "Can We Measure the Individual Prothrombotic or Prohemorrhagic Tendency by Global Coagulation Tests?". Hamostaseologie 40 (3): 364–378. August 2020. doi:10.1055/a-1153-5824. PMID 32726831.
- ↑ "Factor V Leiden thrombophilia". Genet Med 13 (1): 1–16. January 2011. doi:10.1097/GIM.0b013e3181faa0f2. PMID 21116184.
- ↑ 4.0 4.1 4.2 4.3 4.4 4.5 "Oral Contraceptives and Venous Thromboembolism: Focus on Testing that May Enable Prediction and Assessment of the Risk". Semin Thromb Hemost 46 (8): 872–886. November 2020. doi:10.1055/s-0040-1714140. PMID 33080636.
- ↑ 5.00 5.01 5.02 5.03 5.04 5.05 5.06 5.07 5.08 5.09 5.10 5.11 5.12 5.13 5.14 5.15 5.16 5.17 "Combined Oral Contraceptives and Venous Thromboembolism: Review and Perspective to Mitigate the Risk". Front Endocrinol (Lausanne) 12: 769187. 2021. doi:10.3389/fendo.2021.769187. PMID 34956081.
- ↑ "Haemostatic changes in pregnancy". Best Pract Res Clin Haematol 16 (2): 153–68. June 2003. doi:10.1016/s1521-6926(03)00021-5. PMID 12763484.
- ↑ 7.0 7.1 "Changes of hemostasis variables during pregnancy". Semin Vasc Med 3 (1): 13–24. February 2003. doi:10.1055/s-2003-38329. PMID 15199489.
- ↑ 8.0 8.1 "Exogenous hormones, the risk of venous thromboembolism, and activated protein C resistance". Menopause 17 (6): 1099–103. 2010. doi:10.1097/gme.0b013e3181fa264c. PMID 20975607.
- ↑ "Thrombin generation tests". Thromb Res 127 (Suppl 3): S21–5. February 2011. doi:10.1016/S0049-3848(11)70007-X. PMID 21262433.
- ↑ "Global Coagulation Assays in Transgender Women on Oral and Transdermal Estradiol Therapy". J Clin Endocrinol Metab 105 (7): e2369–e2377. July 2020. doi:10.1210/clinem/dgaa262. PMID 32413907.
- ↑ "The measurement and application of thrombin generation". Br J Haematol 130 (5): 653–61. September 2005. doi:10.1111/j.1365-2141.2005.05612.x. PMID 16115120.
- ↑ "Recent Advances in Mainstream Hemostasis Diagnostics and Coagulation Testing". Semin Thromb Hemost 45 (3): 228–246. April 2019. doi:10.1055/s-0038-1676579. PMID 30912101.
- ↑ 13.0 13.1 "Mechanisms of estrogen-induced venous thromboembolism". Thromb Res 126 (1): 5–11. July 2010. doi:10.1016/j.thromres.2010.01.045. PMID 20163835.
- ↑ 14.0 14.1 14.2 14.3 "Effects of non-oral postmenopausal hormone therapy on markers of cardiovascular risk: a systematic review". Fertil Steril 90 (3): 642–72. September 2008. doi:10.1016/j.fertnstert.2007.07.1298. PMID 17923128.
- ↑ 15.0 15.1 "Acquired APC resistance and oral contraceptives: differences between two functional tests". Br J Haematol 105 (1): 88–94. April 1999. doi:10.1111/j.1365-2141.1999.01302.x. PMID 10233368.
- ↑ "Effect of gender-affirming hormone use on coagulation profiles in transmen and transwomen". J Thromb Haemost 19 (4): 1029–1037. April 2021. doi:10.1111/jth.15256. PMID 33527671.
- ↑ 17.0 17.1 "Venous thrombosis and changes of hemostatic variables during cross-sex hormone treatment in transsexual people". J Clin Endocrinol Metab 88 (12): 5723–9. December 2003. doi:10.1210/jc.2003-030520. PMID 14671159.
- ↑ 18.0 18.1 "Venous thrombo-embolism as a complication of cross-sex hormone treatment of male-to-female transsexual subjects: a review". Andrologia 46 (7): 791–5. September 2014. doi:10.1111/and.12150. PMID 23944849.
- ↑ "Risk for Venous Thromboembolism in Transgender Patients Undergoing Cross-Sex Hormone Treatment: A Systematic Review". J Sex Med 18 (7): 1280–1291. July 2021. doi:10.1016/j.jsxm.2021.04.006. PMID 34140253.
- ↑ "Confirmation of the safety of combined oral contraceptives containing oestradiol on the risk of venous thromboembolism". Eur J Contracept Reprod Health Care 27 (2): 83–84. February 2022. doi:10.1080/13625187.2022.2029397. PMID 35133236.
- ↑ 21.0 21.1 Douxfils, Jonathan; Morimont, Laure; Delvigne, Anne-Sophie; Devel, Philippe; Masereel, Bernard; Haguet, Hélène; Bouvy, Céline; Dogné, Jean-Michel (2020-01-28). "Validation and standardization of the ETP-based activated protein C resistance test for the clinical investigation of steroid contraceptives in women: an unmet clinical and regulatory need". Clinical Chemistry and Laboratory Medicine 58 (2): 294–305. doi:10.1515/cclm-2019-0471. ISSN 1437-4331. PMID 31444961. https://pubmed.ncbi.nlm.nih.gov/31444961/.
 |

