Medicine:Environmental enteropathy
| Environmental enteropathy | |
|---|---|
| Other names | Tropical enteropathy or Environmental enteric dysfunction |
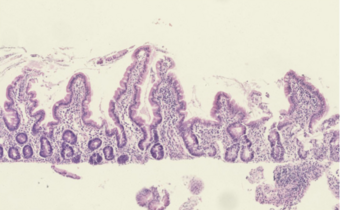 | |
| Histological evidence of enteropathy (inflammatory infiltrate, villus blunting) seen in this intestinal biopsy from a child with malnutrition.[1] | |
| Specialty | Gastroenterology |
| Symptoms | Asymptomatic (most common), altered stool consistency, increased stool frequency, weight loss |
| Complications | Malnutrition, malabsorption, growth stunting, developmental delay, impaired response to oral vaccines |
| Duration | Chronic |
| Causes | Unsanitary food and water sources, fecal-oral contamination, chronic enteric infections, mucosal inflammation |
| Diagnostic method | Intestinal biopsy (gold standard), abnormal sugar absorption test, clinical (significantly more common) |
| Differential diagnosis | Tropical sprue |
| Prevention | Sanitation |
Environmental enteropathy (EE or tropical enteropathy or environmental enteric dysfunction) is an acquired small intestinal disorder characterized by gut inflammation, reduced absorptive surface area in small intestine, and disruption of intestinal barrier function.[2][3][1][4][5] EE is most common amongst children living in low-resource settings.[2][3][1] Acute symptoms are typically minimal or absent.[3] EE can lead to malnutrition, anemia (iron-deficiency anemia and anemia of chronic inflammation),[2] stunted growth, impaired brain development,[6][7][8] and impaired response to oral vaccinations.[9][10]
The cause of EE is multifactorial. Overall, exposure to contaminated food and water leads to a generalized state of intestinal inflammation.[2][3][1] The inflammatory response results in multiple pathological changes to the gastrointestinal tract: Smaller villi, larger crypts (called crypt hyperplasia), increased permeability, and inflammatory cell build-up within the intestines.[3][1][11] These changes result in poor absorption of food, vitamins and minerals.
Standardized, clinically practical diagnostic criteria do not exist. The most accurate diagnostic test is intestinal biopsy. However, this test is invasive and unnecessary for most patients.[2]
Prevention is the strongest and most reliable option for preventing EE and its effects. Therefore, prevention and treatment of EE are often discussed together.[11][12][13]
Signs and symptoms
Environmental enteropathy is believed to result in chronic malnutrition and subsequent growth stunting (low height-for-age measurement) as well as other child development deficits.[5]
Long-term symptoms
- Malnutrition[2][3]
- EE causes malnutrition by way of both malabsorption and nutritional deficiencies.
- Growth and physical development[11]
- The first two years (and the prior 9 months of fetal life) are critical for linear growth. Stunting is an easy to measure symptom of these child development deficits.
- Neurocognitive (brain development)[7][8]
- Effect on oral vaccination[10][14]
- Many oral vaccines, both live and non-living, have proven to be less immunogenic or less protective when administered to infants, children or adults living in low socioeconomic conditions in developing countries than they are when used in industrialized countries. Widespread EE is hypothesized to be a contributing cause for this observation.
Nutrient intake and nutritional status in environmental enteropathy
The relationship between dietary intake and infection is difficult to study since it is reciprocal in nature.[15][16] Further, the gut tissue consumes the nutrients it requires before passage of excess nutrients to the rest of the body.[17][18] The benefits achieved by improved nutrient intake on environmental enteropathy may thus be independent of nutritional status. Nutrient intake during inflammation is usually decreased.[citation needed]
Reports of “poor appetite” by caregivers in LMICs,[19] and restriction of complementary foods during illness[20] is common. Appetite may be reduced both by pro-inflammatory cytokines and leptin[21] and low zinc status,[22] and may be continuous in children with environmental enteropathy.[23] Nutrient availability for growth in environmental enteropathy is further limited due to reduced intestinal surface area and loss of enzymatic activity causing malabsorption of nutrients[24][25] and, following microbial translocation, retention of circulating nutrients (i.e vitamin A, zinc and iron) in body tissues in order to starve pathogens.[23] Associations between nutrient intake and biomarkers for nutrient status[26] and nutrient status and growth[27] are thus likely distorted in children with inflammation.
The systemic inflammation resulting from microbial translocation will increase basal metabolic rate and nutrient needs by the immune system.[28] At the same time, nutrient losses increase due to intestinal secretion.[29] The associations are thus complex, and further complicated by intestinal host-pathogen-microbiome interactions[30] and the effects of these interactions on intestinal nutrient availability,[31][32] where additional research is needed. Finally, evidence of whether nutrition interventions may be successful in children with repeated episodes of infection or persistent subclinical infection is scant.[23] Meanwhile, there seems to be agreement that successful interventions to improve complementary feeding practices[33] and reduce stunting[34][35] must encompass both immediate and underlying causes.
Causes
The development of environmental enteropathy (EE) is multifactorial, but predominantly associated with chronic exposure to contaminated food and water. This is especially true in environments where widespread open defecation and lack of sanitation are common.[2][3][1]
The main cause of environmental enteropathy is likely repeated exposure to enteric pathogens through fecal contamination.[36][35][37] Rotavirus, norovirus, cryptosporidum, shigella, campylobacter and E-coli are among the most prevalent causative agents.[38][39]
Mechanism
Long-term exposure to environmental pathogens leads to a generalized state of intestinal inflammation. Chronic inflammation leads to both functional and structural changes which alter gut permeability and ability of the intestine to absorb nutrients.[2][3][1]
Specifically, structural changes within the intestine include smaller villi, larger crypts (called crypt hyperplasia), increased permeability, and inflammatory cell build-up within the intestines. These changes result in poor absorption of food, vitamins and minerals – or "modest malabsorption".[2][3][1]

Diagnosis
The current gold standard diagnostic test for EE is intestinal biopsy and histological analysis. Histological changes observed include:[1]
- Villous blunting
- Crypt hypertrophy
- Villous fusion
- Mucosal inflammation
The key histological features are villous flattening, crypt hyperplasia and inflammation in the epithelium and lamina propria.[34][40]
However, this procedure is considered too invasive, complex and expensive to be implemented as standard of care.[2] As a result, there are various research efforts underway to identify biomarkers associated with EE, which could serve as less invasive, yet representative, tools to screen for and identify EE from stool samples.[2] In an effort to identify simple, accurate diagnostic tests for EE, the Bill and Melinda Gates Foundation (BMGF) has established an EE biomarkers consortium as part of their Global Grand Challenges initiative (specifically, the Discover Biomarkers of Gut Function challenge).[2]
So far, various biomarkers have been selected and studied based on the current understanding of EE pathophysiology:[2]
- Gut permeability/barrier function
- Intestinal inflammation
- Exocrine (hormonal) markers
- Bacterial translocation markers
- Endotoxin core antibody
- Markers of systemic inflammation
It is postulated that the limited of understanding of EE is partially due to the paucity of reliable biomarkers, making it difficult for researchers to track the epidemiology of the condition and assess the efficacy of interventions.[11] EE is described as a reversible[41][42] condition which is probabilistically associated with poor development, but is neither a necessary nor a sufficient cause and may lead to no observable clinical outcomes.[43] This contributes to difficulties encountered when assessing EE.
Classification
In the 1960s, researchers reported a syndrome of non-specific histopathological and functional changes to the small intestine in individuals living in unsanitary conditions.[3] This syndrome was observed predominantly in tropical regions across Latin America, sub-Saharan Africa and Asia. The geographic distribution of the syndrome lead to the original term of "tropical enteropathy" (sometimes also "tropical jejunopathy").[3]
Following initial reports, further investigation revealed that these symptoms were not specific to tropical climates. For example, individuals in more wealthy tropical countries, such as Qatar and Singapore, did not exhibit these symptoms.[1] Similarly, subsequent studies have shown this condition to be common across the developing world, closely associated with impoverished conditions but independent of climate or geography.[1][11] As a result, the term "environmental enteropathy" was introduced to specify that this condition is not only found in tropical areas and is believed to be caused by environmental factors.[3]
Prevention

Prevention focuses on improving access to improved water, sanitation and hygiene (WASH).[13][6] Another important factor responsible for EE is contaminated soil in child play spaces, often caused by the presence of livestock such as chicken in the household. Creating a clean play space might therefore be an effective preventive measure for EE in toddlers.[44] Some potential strategies to prevent EE are:
- Improve Water, Sanitation and Hygiene practice both at individual and household levels
- Reduce faecal contamination of food and water
- Limit exposure to livestock and poultry
Treatment
There is no effective and accepted treatment for EE. Treatment focuses on addressing the central components of intestinal inflammation, bacterial overgrowth and nutritional supplementation.[2] Some potential interventions to improve symptoms associated with EE are:
- Exclusive breastfeeding[45]
- Improve dietary diversity
- Use of prebiotics, probiotics, and synbiotics
- Dietary interventions such as egg and milk
- Multiple micronutrient supplements[46]
- Supplementation with lactoferrin and lysozyme[47]
- Antimicrobials in the context of acute malnutrition and infection
The role of nutrition in environmental enteropathy is increasingly being recognized.[43] Environmental enteropathy is likely associated with energy deficiency and underweight. Mice fed a moderately energy- and protein deficient diet who are exposed to intestinal pathogens show traits similar to environmental enteropathy.[48] Further, weight gain in malnourished children is shown to improve environmental enteropathy.[49] Severe malnourishment is also likely associated with microbiota immaturity,[50] which might increase environmental enteropathy.[51] The intestinal mucosa turnover is dynamic, nutrient-dependent and rapid,[52] and malnourished children have rate-limiting stores for repairing mucosal damage.[24]
The nutrients known to contribute to intestinal regeneration and improved barrier function are sulphur containing amino acids, [53] glutamine, vitamin A and zinc.[35][52] Meanwhile, studies investigating associations between glutamine[54] or vitamin A supplementation,[55][17] serum retinol[56][57] or zinc supplementation either alone,[58] in combination with vitamin A[59] or with micronutrients and antibiotics[60] and environmental enteropathy show mixed results.
Gut barrier repair and gut function may also be improved by a reduction in the inflammatory response. Short-chain fatty acids (SCFA) result from fermentation of non starch polysaccharides in the colon.[52] It is likely that short-chain fatty acids in addition to zinc[52] and polyunsaturated fatty acids (PUFAs)[61] may reduce gastrointestinal inflammation. Although neither fibre nor polyunsaturated fatty acids provided as supplements improved lactulose:mannitol (L:M) ratio or inflammation in intervention trials,[62][63] an increased protein and fibre intake from legumes as complementary food, might improve environmental enteropathy.[64][65] Cessation of breastfeeding and introduction of complementary foods, especially foods with high fibre and protein content, also likely increases microbiota diversity,[66] which might benefit the intestine. As for micronutrient intake and environmental enteropathy, studies from Africa have demonstrated that multiple micronutrient supplementation may improve lactulose:mannitol (L:M) ratio in adults,[67] and transiently in children.[68] Finally, despite the diverse roles attributed to zinc in environmental enteropathy the effect of supplementation as prophylaxis is uncertain.[69] This may partly be due to the perturbed nutrient metabolism occurring in environmental enteropathy.[citation needed]
Epidemiology
Environmental enteropathy (EE) primarily affects children living in low- and middle-income countries (LMICs).[70] Children living in these countries were found to have enteric pathogens related to EE in their systems throughout much of their early childhoods.[70] Gastrointestinal abnormalities associated with EE are not congenital but are acquired during infancy and persist into adulthood.[71][72] Such abnormalities tend to develop after the first semester of life and are not present in newborns.[71]
Historically, environmental enteropathy has been prevalent in LMICs.[72] The geographic distribution of environmental enteropathy has shown an increase in incidence in such areas of poor sanitation and hygiene.[70] EE was first described in studies from the 1960-70s conducted in Asia, Africa, the Indian subcontinent, and Central America, during which it was discovered that signs of EE were high among otherwise healthy adults and children.[73] A study from 1971 following US Peace Corps volunteers is often cited as being the first study to demonstrate the ability to acquire and recover from EE according to the environment.[72] Participants experienced symptoms of chronic enteric infection during and shortly after returning from their placement in low- and middle-income countries.[70] Symptoms experienced by those abroad were resolved within one to two years after returning home to the US.[72] These results lead to the suggestion of the environment being a cause of EE, and a later study in Zambia was able to draw similar conclusions.[72] By the early 1990s, environmental enteropathy was found to be a widespread problem affecting infants and children.[72] Today, enteric infections and diarrheal diseases like environmental enteropathy account for 760,000 deaths per year worldwide, making EE the second leading cause of death in children under five years old.[73]
The exact causes and consequences of EE have been difficult to establish due, in part, to the lack of a clear disease definition.[70] However, risk factors do exist and they can be both environmental and nutritional.[70] Preexisting conditions such as micronutrient deficiencies, diarrheal diseases, and various chronic infections all serve as risk factors for EE.[70] Environmental conditions such as poor sanitation and unimproved water sources also contribute to the prevalence of EE.[70] Exposure to environmental microbial agents such as these is thought to be the most important factor in the development of EE.[71]
Research initiatives
There are multiple large-field, multi-country research initiatives focusing on strategies to prevent and treat EE.[11]
- The MAL-ED project
- The Bangladesh Environmental Enteric Dysfunction (BEED) Study (ClinicalTrials.gov Identifier: NCT02812615)
- The Study of Environmental Enteropathy and Malnutrition (SEEM) in Pakistan (ClinicalTrials.gov Identifier: NCT03588013)
- The Alive and Thrive nutrition project
- The Sanitation, Hygiene and Infant Nutrition Efficacy (SHINE) Trial (ClinicalTrials.gov identifier: NCT01824940)
- The WASH Benefits Study
References
![]() This article incorporates text by Marianne Sandsmark Morseth available under the CC BY-SA 3.0 license.
This article incorporates text by Marianne Sandsmark Morseth available under the CC BY-SA 3.0 license.
- ↑ 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 1.11 "Tropical Enteropathies". Current Gastroenterology Reports 19 (7): 29. July 2017. doi:10.1007/s11894-017-0570-0. PMID 28540669.
- ↑ 2.00 2.01 2.02 2.03 2.04 2.05 2.06 2.07 2.08 2.09 2.10 2.11 2.12 2.13 "Environmental enteropathy". Current Opinion in Gastroenterology 32 (1): 12–17. January 2016. doi:10.1097/MOG.0000000000000226. PMID 26574871.
- ↑ 3.00 3.01 3.02 3.03 3.04 3.05 3.06 3.07 3.08 3.09 3.10 3.11 "Environmental enteropathy: critical implications of a poorly understood condition". Trends in Molecular Medicine 18 (6): 328–336. June 2012. doi:10.1016/j.molmed.2012.04.007. PMID 22633998.
- ↑ "Pathophysiology of environmental enteric dysfunction and its impact on oral vaccine efficacy". Mucosal Immunology 11 (5): 1290–1298. September 2018. doi:10.1038/s41385-018-0036-1. PMID 29988114.
- ↑ 5.0 5.1 "Environmental Enteric Dysfunction: A Case Definition for Intervention Trials". The American Journal of Tropical Medicine and Hygiene 97 (6): 1643–1646. December 2017. doi:10.4269/ajtmh.17-0183. PMID 29016294.
- ↑ 6.0 6.1 "Water, sanitation, and hygiene (WASH), environmental enteropathy, nutrition, and early child development: making the links". Annals of the New York Academy of Sciences 1308 (1): 118–128. January 2014. doi:10.1111/nyas.12330. PMID 24571214. Bibcode: 2014NYASA1308..118N.
- ↑ 7.0 7.1 "Neurodevelopment, Nutrition, and Inflammation: The Evolving Global Child Health Landscape". Pediatrics 139 (Suppl 1): S12–S22. April 2017. doi:10.1542/peds.2016-2828d. PMID 28562245.
- ↑ 8.0 8.1 "Neurodevelopment: The Impact of Nutrition and Inflammation During Early to Middle Childhood in Low-Resource Settings". Pediatrics 139 (Suppl 1): S59–S71. April 2017. doi:10.1542/peds.2016-2828h. PMID 28562249.
- ↑ "Early-life enteric infections: relation between chronic systemic inflammation and poor cognition in children". Nutrition Reviews 74 (6): 374–386. June 2016. doi:10.1093/nutrit/nuw008. PMID 27142301.
- ↑ 10.0 10.1 "Vaccines against enteric infections for the developing world". Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences 370 (1671): 20150142. June 2015. doi:10.1098/rstb.2015.0142. PMID 25964464.
- ↑ 11.0 11.1 11.2 11.3 11.4 11.5 "Enteropathies in the developing world: neglected effects on global health". The American Journal of Tropical Medicine and Hygiene 86 (5): 756–763. May 2012. doi:10.4269/ajtmh.2012.11-0743. PMID 22556071.
- ↑ "Child undernutrition, tropical enteropathy, toilets, and handwashing". Lancet 374 (9694): 1032–1035. September 2009. doi:10.1016/s0140-6736(09)60950-8. PMID 19766883.
- ↑ 13.0 13.1 "Preventing environmental enteric dysfunction through improved water, sanitation and hygiene: an opportunity for stunting reduction in developing countries". Maternal & Child Nutrition 12 (Suppl 1): 106–120. May 2016. doi:10.1111/mcn.12220. PMID 26542185.
- ↑ "Exploring the role of environmental enteropathy in malnutrition, infant development and oral vaccine response". Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences 370 (1671): 20140143. June 2015. doi:10.1098/rstb.2014.0143. PMID 25964455.
- ↑ Scrimshaw NS. Historical concepts of interactions, synergism and antagonism between nutrition and infection. J Nutr 2003; 133(1): 316S-21S.
- ↑ Solomons NW. Malnutrition and infection: an update. The British journal of nutrition 2007; 98 Suppl 1: S5-10.
- ↑ 17.0 17.1 Thurnham DI, Northrop-Clewes CA, McCullough FS, Das BS, Lunn PG. Innate immunity, gut integrity, and vitamin A in Gambian and Indian infants. J Infect Dis 2000; 182 Suppl 1: S23-8.
- ↑ Van Der Schoor SR, Reeds PJ, Stoll B, et al. The high metabolic cost of a functional gut. Gastroenterology 2002; 123(6): 1931-40.
- ↑ Brown KH, Peerson JM, Lopez de Romana G, de Kanashiro HC, Black RE. Validity and epidemiology of reported poor appetite among Peruvian infants from a low-income, periurban community. The American journal of clinical nutrition 1995; 61(1): 26-32.
- ↑ Paintal K, Aguayo VM. Feeding practices for infants and young children during and after common illness. Evidence from South Asia. Maternal & child nutrition 2016; 12 Suppl 1: 39-71.
- ↑ Somech R, Reif S, Golander A, Spirer Z. Leptin and C-reactive protein levels correlate during minor infection in children. Isr Med Assoc J 2007; 9(2): 76-8.
- ↑ Prasad AS. Clinical and biochemical manifestations of zinc deficiency in human subjects. Journal of the American College of Nutrition 1985; 4(1): 65-72.
- ↑ 23.0 23.1 23.2 Dewey KG, Mayers DR. Early child growth: how do nutrition and infection interact? Maternal & child nutrition 2011; 7 Suppl 3: 129-42.
- ↑ 24.0 24.1 Guerrant RL, Oria RB, Moore SR, Oria MO, Lima AA. Malnutrition as an enteric infectious disease with long-term effects on child development. Nutrition reviews 2008; 66(9): 487-505.
- ↑ Trehan I, Kelly P, Shaikh N, Manary MJ. New insights into environmental enteric dysfunction. Arch Dis Child 2016; 101(8): 741-4.
- ↑ Martin-Prevel Y, Allemand P, Nikiema L, et al. Biological Status and Dietary Intakes of Iron, Zinc and Vitamin A among Women and Preschool Children in Rural Burkina Faso. PloS one 2016; 11(3): e0146810.
- ↑ Ahmed T, Auble D, Berkley JA, et al. An evolving perspective about the origins of childhood undernutrition and nutritional interventions that includes the gut microbiome. Ann N Y Acad Sci 2014; 1332: 22-38.
- ↑ Syed S, Ali A, Duggan C. Environmental Enteric Dysfunction in Children. J Pediatr Gastroenterol Nutr 2016; 63(1): 6-14.
- ↑ Krebs NF, Miller LV, Hambidge KM. Zinc deficiency in infants and children: a review of its complex and synergistic interactions. Paediatrics and international child health 2014; 34(4): 279-88.
- ↑ Prendergast A, Kelly P. Enteropathies in the developing world: neglected effects on global health. Am J Trop Med Hyg 2012; 86(5): 756-63.
- ↑ Yatsunenko T, Rey FE, Manary MJ, et al. Human gut microbiome viewed across age and geography. Nature 2012; 486(7402): 222-7.
- ↑ Biesalski HK. Nutrition meets the microbiome: micronutrients and the microbiota. Ann N Y Acad Sci 2016; 1372(1): 53-64.
- ↑ Stewart CP, Iannotti L, Dewey KG, Michaelsen KF, Onyango AW. Contextualising complementary feeding in a broader framework for stunting prevention. Maternal & child nutrition 2013; 9 Suppl 2: 27-45.
- ↑ 34.0 34.1 Owino V, Ahmed T, Freemark M, et al. Environmental Enteric Dysfunction and Growth Failure/Stunting in Global Child Health. Pediatrics 2016; 138(6).
- ↑ 35.0 35.1 35.2 McKay S, Gaudier E, Campbell DI, Prentice AM, Albers R. Environmental enteropathy: new targets for nutritional interventions. Int Health 2010; 2(3): 172-80.
- ↑ MAL-ED Network Investigators. Childhood stunting in relation to the pre- and postnatal environment during the first 2 years of life: The MAL-ED longitudinal birth cohort study. PLOS Medicine 2017.
- ↑ George CM, Burrowes V, Perin J, et al. Enteric Infections in Young Children are Associated with Environmental Enteropathy and Impaired Growth. Trop Med Int Health 2018; 23(1): 26-33.
- ↑ Prendergast AJ, Kelly P. Interactions between intestinal pathogens, enteropathy and malnutrition in developing countries. Curr Opin Infect Dis 2016; 29(3): 229-36.
- ↑ Platts-Mills JA, Babji S, Bodhidatta L, et al. Pathogen-specific burdens of community diarrhoea in developing countries: a multisite birth cohort study (MAL-ED). Lancet Glob Health 2015; 3(9): e564-75.
- ↑ Crane RJ, Jones KD, Berkley JA. Environmental enteric dysfunction: an overview. Food Nutr Bull 2015; 36(1 Suppl): S76-87.
- ↑ Lindenbaum J, Gerson CD, Kent TH. Recovery of small-intestinal structure and function after residence in the tropics. I. Studies in Peace Corps volunteers. Ann Intern Med 1971; 74(2): 218-22.
- ↑ Kosek M, Guerrant RL, Kang G, et al. Assessment of environmental enteropathy in the MAL-ED cohort study: theoretical and analytic framework. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America 2014; 59 Suppl 4: S239-47.
- ↑ 43.0 43.1 Rogawski ET, Guerrant RL. The Burden of Enteropathy and "Subclinical" Infections. Pediatr Clin North Am 2017; 64(4): 815-36.
- ↑ "Enteric Infections in Young Children are Associated with Environmental Enteropathy and Impaired Growth". Tropical Medicine & International Health 23 (1): 26–33. January 2018. doi:10.1111/tmi.13002. PMID 29121442.
- ↑ "Cessation of exclusive breastfeeding and seasonality, but not small intestinal bacterial overgrowth, are associated with environmental enteric dysfunction: A birth cohort study amongst infants in rural Kenya". eClinicalMedicine 47: 101403. May 2022. doi:10.1016/j.eclinm.2022.101403. PMID 35497062.
- ↑ "Multiple micronutrient supplementation transiently ameliorates environmental enteropathy in Malawian children aged 12-35 months in a randomized controlled clinical trial". The Journal of Nutrition 144 (12): 2059–2065. December 2014. doi:10.3945/jn.114.201673. PMID 25411039.
- ↑ "Supplementation With Lactoferrin and Lysozyme Ameliorates Environmental Enteric Dysfunction: A Double-Blind, Randomized, Placebo-Controlled Trial". The American Journal of Gastroenterology 114 (4): 671–678. April 2019. doi:10.14309/ajg.0000000000000170. PMID 30829679.
- ↑ Brown EM, Wlodarska M, Willing BP, et al. Diet and specific microbial exposure trigger features of environmental enteropathy in a novel murine model. Nat Commun 2015; 6: 7806.
- ↑ Hossain MI, Nahar B, Hamadani JD, Ahmed T, Roy AK, Brown KH. Intestinal mucosal permeability of severely underweight and nonmalnourished Bangladeshi children and effects of nutritional rehabilitation. J Pediatr Gastroenterol Nutr 2010; 51(5): 638-44.
- ↑ Subramanian S, Huq S, Yatsunenko T, et al. Persistent gut microbiota immaturity in malnourished Bangladeshi children. Nature 2014; 510(7505): 417-21.
- ↑ Buffie CG, Pamer EG. Microbiota-mediated colonization resistance against intestinal pathogens. Nat Rev Immunol 2013; 13(11): 790-801.
- ↑ 52.0 52.1 52.2 52.3 Ziegler TR, Evans ME, Fernandez-Estivariz C, Jones DP. Trophic and cytoprotective nutrition for intestinal adaptation, mucosal repair, and barrier function. Annu Rev Nutr 2003; 23: 229-61.
- ↑ Bickler SW, Ring J, De Maio A. Sulfur amino acid metabolism limits the growth of children living in environments of poor sanitation. Med Hypotheses 2011; 77(3): 380-2.
- ↑ Williams EA, Elia M, Lunn PG. A double-blind, placebo-controlled, glutamine-supplementation trial in growth-faltering Gambian infants. The American journal of clinical nutrition 2007; 86(2): 421-7.
- ↑ Lima AA, Soares AM, Lima NL, et al. Effects of vitamin A supplementation on intestinal barrier function, growth, total parasitic, and specific Giardia spp infections in Brazilian children: a prospective randomized, double-blind, placebo-controlled trial. J Pediatr Gastroenterol Nutr 2010; 50(3): 309-15.
- ↑ Hossain MI, Haque R, Mondal D, et al. Undernutrition, Vitamin A and Iron Deficiency Are Associated with Impaired Intestinal Mucosal Permeability in Young Bangladeshi Children Assessed by Lactulose/Mannitol Test. PloS one 2016; 11(12): e0164447.
- ↑ Vieira MM, Paik J, Blaner WS, et al. Carotenoids, retinol, and intestinal barrier function in children from northeastern Brazil. J Pediatr Gastroenterol Nutr 2008; 47(5): 652-9.
- ↑ Ryan KN, Stephenson KB, Trehan I, et al. Zinc or albendazole attenuates the progression of environmental enteropathy: a randomized controlled trial. Clin Gastroenterol Hepatol 2014; 12(9): 1507-13 e1.
- ↑ Chen P, Soares AM, Lima AA, et al. Association of vitamin A and zinc status with altered intestinal permeability: analyses of cohort data from northeastern Brazil. J Health Popul Nutr 2003; 21(4): 309-15.
- ↑ Wang AZ, Shulman RJ, Crocker AH, et al. A Combined Intervention of Zinc, Multiple Micronutrients, and Albendazole Does Not Ameliorate Environmental Enteric Dysfunction or Stunting in Rural Malawian Children in a Double-Blind Randomized Controlled Trial. J Nutr 2017; 147(1): 97-103.
- ↑ Teitelbaum JE, Allan Walker W. Review: the role of omega 3 fatty acids in intestinal inflammation. J Nutr Biochem 2001; 12(1): 21-32.
- ↑ Ordiz MI, May TD, Mihindukulasuriya K, et al. The effect of dietary resistant starch type 2 on the microbiota and markers of gut inflammation in rural Malawi children. Microbiome 2015; 3: 37.
- ↑ van der Merwe LF, Moore SE, Fulford AJ, et al. Long-chain PUFA supplementation in rural African infants: a randomized controlled trial of effects on gut integrity, growth, and cognitive development. The American journal of clinical nutrition 2013; 97(1): 45-57.
- ↑ Agapova SE, Stephenson KB, Divala O, et al. Additional Common Bean in the Diet of Malawian Children Does Not Affect Linear Growth, but Reduces Intestinal Permeability. J Nutr 2018; 148(2): 267-74.
- ↑ Stephenson KB, Agapova SE, Divala O, et al. Complementary feeding with cowpea reduces growth faltering in rural Malawian infants: a blind, randomized controlled clinical trial. The American journal of clinical nutrition 2017; 106(6): 1500-7.
- ↑ Laursen MF, Bahl MI, Michaelsen KF, Licht TR. First Foods and Gut Microbes. Front Microbiol 2017; 8: 356.
- ↑ Louis-Auguste J, Greenwald S, Simuyandi M, Soko R, Banda R, Kelly P. High dose multiple micronutrient supplementation improves villous morphology in environmental enteropathy without HIV enteropathy: results from a double-blind randomised placebo controlled trial in Zambian adults. BMC Gastroenterol 2014; 14: 15.
- ↑ Smith HE, Ryan KN, Stephenson KB, et al. Multiple micronutrient supplementation transiently ameliorates environmental enteropathy in Malawian children aged 12-35 months in a randomized controlled clinical trial. J Nutr 2014; 144(12): 2059-65.
- ↑ Kulkarni H, Mamtani M, Patel A. Roles of zinc in the pathophysiology of acute diarrhea. Curr Infect Dis Rep 2012; 14(1): 24-32.
- ↑ 70.0 70.1 70.2 70.3 70.4 70.5 70.6 70.7 "Environmental enteric dysfunction: a review of potential mechanisms, consequences and management strategies". BMC Medicine 17 (1): 181. November 2019. doi:10.1186/s12916-019-1417-3. PMID 31760941.
- ↑ 71.0 71.1 71.2 "Environmental enteric dysfunction and growth". Jornal de Pediatria 95: 85–94. 2019-03-01. doi:10.1016/j.jped.2018.11.004. PMID 30629923.
- ↑ 72.0 72.1 72.2 72.3 72.4 72.5 "Environmental enteric dysfunction: an overview". Food and Nutrition Bulletin 36 (1 Suppl): S76–S87. March 2015. doi:10.1177/15648265150361S113. PMID 25902619.
- ↑ 73.0 73.1 "Environmental Enteric Dysfunction in Children". Journal of Pediatric Gastroenterology and Nutrition 63 (1): 6–14. July 2016. doi:10.1097/MPG.0000000000001147. PMID 26974416.
 |
