Physics:Dication
A dication is any cation, of general formula X2+, formed by the removal of two electrons from a neutral species.
Diatomic dications corresponding to stable neutral species (e.g. H2+2 formed by removal of two electrons from H2) often decay quickly into two singly charged particles (H+), due to the loss of electrons in bonding molecular orbitals. Energy levels of diatomic dications can be studied with good resolution by measuring the yield of pairs of zero-kinetic-energy electrons from double photoionization of a molecule as a function of the photoionizing wavelength (threshold photoelectrons coincidence spectroscopy – TPEsCO). The He2+2 dication is kinetically stable.
An example of a stable diatomic dication which is not formed by oxidation of a neutral diatomic molecule is the dimercury dication Hg2+2. An example of a polyatomic dication is S2+8, formed by oxidation of S8 and unstable with respect to further oxidation over time to form SO2.
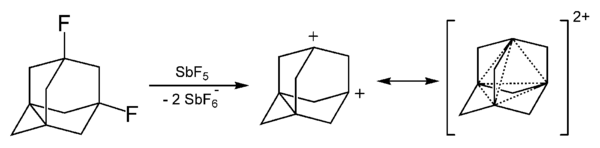
Many organic dications can be detected in mass spectrometry for example CH2+4 (a CH2+2·H2 complex) and the acetylene dication C2H2+2.[1] The adamantyl dication has been synthesized.[2]
Divalent metals
Some metals are commonly found in the form of dications when in the form of salts, or dissolved in water. Examples include the alkaline earth metals (Be2+, Mg2+, Ca2+, Sr2+, Ba2+, Ra2+); later 3d transition metals (V2+, Cr2+, Mn2+, Fe2+, Co2+, Ni2+, Cu2+, Zn2+); group 12 elements (Zn2+, Cd2+, Hg2+); and the heavy members of the carbon group (Sn2+, Pb2+).
Presence in space
Multiply-charged atoms are quite common in the Solar System in the so-called Solar wind. Among these, the most abundant dication is He2+ (an alpha particle). However, molecular dications, in particular CO22+, have never been observed so far though predicted to be present for instance at Mars.[3] Indeed, this ion by means of its symmetry and strong double bounds is more stable (longer lifetime) than other dications. In 2020, the molecular dication CO22+ has been confirmed to be present in the atmosphere of Mars[4] and around Comet 67P.[5]
References
- ↑ Lammertsma, K.; von Ragué Schleyer, P.; Schwarz, H. (1989). "Organic Dications: Gas Phase Experiments and Theory in Concert". Angew. Chem. Int. Ed. Engl. 28 (10): 1321–1341. doi:10.1002/anie.198913211.
- ↑ Bremer, Matthias; von Ragué Schleyer, Paul; Schötz, Karl; Kausch, Michael; Schindler, Michael (August 1987). "Four-Center Two-Electron Bonding in a Tetrahedral Topology. Experimental Realization of Three-Dimensional Homoaromaticity in the 1,3-Dehydro-5,7-adamantanediyl Dication" (in en). Angewandte Chemie International Edition in English 26 (8): 761–763. doi:10.1002/anie.198707611. ISSN 0570-0833. https://onlinelibrary.wiley.com/doi/abs/10.1002/anie.198707611.
- ↑ Witasse, O.; Dutuit, O.; Lilensten, J.; Thissen, R.; Zabka, J.; Alcaraz, C.; Blelly, P.-L.; Bougher, S. W. et al. (2020). "Prediction of a CO22+ layer in the atmosphere of Mars". Geophysical Research Letters 29 (8): 104-1-104-4. doi:10.1029/2002GL014781.
- ↑ Gu, H.; Cui, J.; Diu, D.; Dai, L.; Huang, J.; Wu, X.; Hao, Y.; Wei, Y. (2020). "Observation of CO22+ dication in the dayside Martian upper atmosphere". Earth and Planetary Physics 4 (4): 396–402. doi:10.26464/epp2020036.
- ↑ Beth, A.; Altwegg, K.; Balsiger, H.; Berthelier, J.-J.; Combi, M. R.; De Keyser, J.; Fiethe, B.; Fuselier, S.A. et al. (2020). "ROSINA ion zoo at Comet 67P". Astronomy and Astrophysics 642 (October 2020): A27. doi:10.1051/0004-6361/201936775.
 |
