Physics:Diffusionless transformation

This article has multiple issues. Please help improve it or discuss these issues on the talk page. (Learn how and when to remove these template messages)
(Learn how and when to remove this template message)No issues specified. Please specify issues, or remove this template. |
A diffusionless transformation, commonly known as displacive transformation, denotes solid-state alterations in crystal structures that do not hinge on the diffusion of atoms across extensive distances. Rather, these transformations manifest as a result of synchronized shifts in atomic positions, wherein atoms undergo displacements of distances smaller than the spacing between adjacent atoms, all while preserving their relative arrangement. An example of such a phenomenon is the martensitic transformation, a notable occurrence observed in the context of steel materials.
The term "martensite" was originally coined to describe the rigid and finely dispersed constituent that emerges in steels subjected to rapid cooling. Subsequent investigations revealed that materials beyond ferrous alloys, such as non-ferrous alloys and ceramics, can also undergo diffusionless transformations. Consequently, the term "martensite" has evolved to encompass the resultant product arising from such transformations in a more inclusive manner. In the context of diffusionless transformations, a cooperative and homogeneous movement occurs, leading to a modification in the crystal structure during a phase change. These movements are small, usually less than their interatomic distances, and the neighbors of an atom remain close.
The systematic movement of large numbers of atoms led some to refer to them as military transformations, in contrast to civilian diffusion-based phase changes, initially by Charles Frank and John Wyrill Christian.[1][2]
The most commonly encountered transformation of this type is the martensitic transformation, which is probably the most studied but is only one subset of non-diffusional transformations. The martensitic transformation in steel represents the most economically significant example of this category of phase transformations. However, an increasing number of alternatives, such as shape memory alloys, are becoming more important as well.
Classification and definitions
The phenomenon in which atoms or groups of atoms coordinate to displace their neighboring counterparts resulting in structural modification is known as a displacive transformation. The scope of displacive transformations is extensive, encompassing a diverse array of structural changes. As a result, additional classifications have been devised to provide a more nuanced understanding of these transformations.[3]
The first distinction can be drawn between transformations dominated by lattice-distortive strains and those where shuffles are of greater importance.
Homogeneous lattice-distortive strains, also known as Bain strains, transform one Bravais lattice into a different one. This can be represented by a strain matrix S which transforms one vector, y, into a new vector, x:
This is homogeneous, as straight lines are transformed into new straight lines. Examples of such transformations include a cubic lattice increasing in size on all three axes (dilation) or shearing into a monoclinic structure.
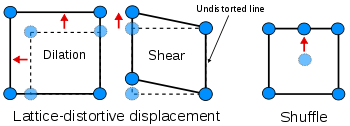
Shuffles, aptly named, refer to the minute displacement of atoms within the unit cell. Notably, pure shuffles typically do not induce a modification in the shape of the unit cell; instead, they predominantly impact its symmetry and overall structural configuration.
Phase transformations typically give rise to the formation of an interface delineating the transformed and parent materials. The energy requisite for establishing this new interface is contingent upon its characteristics, specifically how well the two structures interlock. An additional energy consideration arises when the transformation involves a change in shape. In such instances, if the new phase is constrained by the surrounding material, elastic or plastic deformation may occur, introducing a strain energy term. The interplay between these interfacial and strain energy terms significantly influences the kinetics of the transformation and the morphology of the resulting phase. Notably, in shuffle transformations characterized by minimal distortions, interfacial energies tend to predominate, distinguishing them from lattice-distortive transformations where the impact of strain energy is more pronounced.
A subclassification of lattice-distortive displacements can be made by considering the dilutional and shear components of the distortion. In transformations dominated by the shear component, it is possible to find a line in the new phase that is undistorted from the parent phase while all lines are distorted when the dilation is predominant. Shear-dominated transformations can be further classified according to the magnitude of the strain energies involved compared to the innate vibrations of the atoms in the lattice and hence whether the strain energies have a notable influence on the kinetics of the transformation and the morphology of the resulting phase. If the strain energy is a significant factor, then the transformations are dubbed martensitic, if not the transformation is referred to as quasi-martensitic.
Iron-carbon martensitic transformation
The distinction between austenitic and martensitic steels is subtle in nature.[4] Austenite exhibits a face-centered cubic (FCC) unit cell, whereas the transformation to martensite entails a distortion of this cube into a body-centered tetragonal shape (BCT). This transformation occurs due to a displacive process, where interstitial carbon atoms lack the time to diffuse out.[5] Consequently, the unit cell undergoes a slight elongation in one dimension and contraction in the other two. Despite differences in the symmetry of the crystal structures, the chemical bonding between them remains similar.
The iron-carbon martensitic transformation generates an increase in hardness. The martensitic phase of the steel is supersaturated in carbon and thus undergoes solid solution strengthening.[6] Similar to work-hardened steels, defects prevent atoms from sliding past one another in an organized fashion, causing the material to become harder.
Pseudo martensitic transformation
In addition to displacive transformation and diffusive transformation, a new type of phase transformation that involves a displacive sublattice transition and atomic diffusion was discovered using a high-pressure X-ray diffraction system.[7] The new transformation mechanism has been christened pseudo martensitic transformation.[8]
References
Notes
- ↑ D.A. Porter and K.E. Easterling, Phase transformations in metals and alloys, Chapman & Hall, 1992, p.172 ISBN 0-412-45030-5
- ↑ 西山 善次 (1967). "マルテンサイトの格子欠陥" (in Japanese). 日本金属学会会報 (日本金属学会) 6 (7): 497–506. doi:10.2320/materia1962.6.497. ISSN 1884-5835. https://www.jstage.jst.go.jp/article/materia1962/6/7/6_7_497/_article/-char/ja.
- ↑ Cohen, Morris; Olson, G. B.; Clapp, P. C. (1979). "On the Classification of Displacive Phase Transformations". International Conference on Martensitic Transformations. pp. 1–11. https://www.phase-trans.msm.cam.ac.uk/2009/ICOMAT79/1.pdf.
- ↑ Duhamel, C.; Venkataraman, S.; Scudino, S.; Eckert, J. (May 2008), "Diffusionless transformations", Basics of Thermodynamics and Phase Transitions in Complex Intermetallics, Book Series on Complex Metallic Alloys (WORLD SCIENTIFIC) 1: pp. 119–145, doi:10.1142/9789812790590_0006, ISBN 978-981-279-058-3, Bibcode: 2008btpt.book..119D, https://www.worldscientific.com/doi/abs/10.1142/9789812790590_0006, retrieved 2023-08-11
- ↑ Shewmon, Paul G. (1969) (in en). Transformations in Metals. New York: McGraw-Hill. p. 333. ISBN 978-0-07-056694-1.
- ↑ Banerjee, S.; Mukhopadhyay, P. (2007). Phase transformations: examples from titanium and zirconium alloys. Pergamon materials series. Amsterdam ; Oxford: Elsevier/Pergamon. ISBN 978-0-08-042145-2. OCLC 156890507. https://www.worldcat.org/title/156890507.
- ↑ Chen, Jiuhua; Weidner, Donald J.; Parise, John B.; Vaughan, Michael T.; Raterron, Paul (2001-04-30). "Observation of Cation Reordering during the Olivine-Spinel Transition in Fayalite by In Situ Synchrotron X-Ray Diffraction at High Pressure and Temperature". Physical Review Letters (American Physical Society (APS)) 86 (18): 4072–4075. doi:10.1103/physrevlett.86.4072. ISSN 0031-9007. PMID 11328098. Bibcode: 2001PhRvL..86.4072C. https://link.aps.org/doi/10.1103/PhysRevLett.86.4072.
- ↑ Leutwyler, Kristin (May 2, 2001). "New Phase Transition May Explain Deep Earthquakes". https://www.scientificamerican.com/article/new-phase-transition-may/.
Bibliography
- Christian, J.W., Theory of Transformations in Metals and Alloys, Pergamon Press (1975)
- Khachaturyan, A.G., Theory of Structural Transformations in Solids, Dover Publications, NY (1983)
- Green, D.J.; Hannick, R.; Swain, M.V. (1989). Transformation Toughening of Ceramics. Boca Raton: CRC Press. ISBN 0-8493-6594-5.
External links
- Extensive resources from Cambridge University
- The cubic-to-tetragonal transition
- European Symposium on Martensitic Transformations (ESOMAT)
- PTC Lab for martensite crystallography
 |
