Physics:Lateral flow test
Lateral flow tests,[1] also known as lateral flow immunochromatographic assays, are simple devices intended to detect the presence of a target substance in a liquid sample without the need for specialized and costly equipment. These tests are widely used in medical diagnostics for home testing, point of care testing, or laboratory use. For instance, the home pregnancy test is a lateral flow test that detects a certain hormone. These tests are simple, economic and generally show results in around 5-30 minutes.[2] Many lab-based applications increase the sensitivity of simple lateral flow tests by employing additional dedicated equipment.[3]
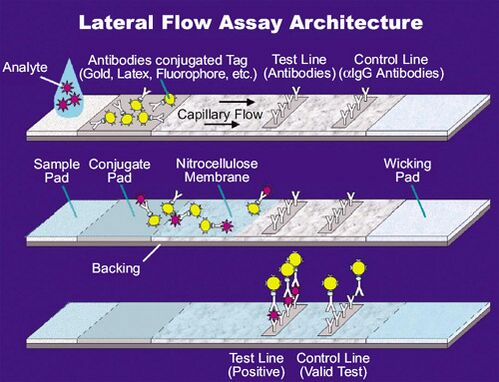
Lateral flow tests operate on the same principles as the enzyme-linked immunosorbent assays (ELISA). In essence, these tests run the liquid sample along the surface of a pad with reactive molecules that show a visual positive or negative result. The pads are based on a series of capillary beds, such as pieces of porous paper,[4] microstructured polymer,[5][6] or sintered polymer.[7][failed verification] Each of these pads has the capacity to transport fluid (e.g., urine, blood, saliva) spontaneously.
The sample pad acts as a sponge and holds an excess of sample fluid. Once soaked, the fluid flows to the second conjugate pad in which the manufacturer has stored freeze dried bio-active particles called conjugates (see below) in a salt-sugar matrix. The conjugate pad contains all the reagents required for an optimized chemical reaction between the target molecule (e.g., an antigen) and its chemical partner (e.g., antibody) that has been immobilized on the particle's surface. This marks target particles as they pass through the pad and continue across to the test and control lines. The test line shows a signal, often a color as in pregnancy tests. The control line contains affinity ligands which show whether the sample has flowed through and the bio-molecules in the conjugate pad are active. After passing these reaction zones, the fluid enters the final porous material, the wick, that simply acts as a waste container.
Lateral flow tests can operate as either competitive or sandwich assays.
Colored particles
In principle, any colored particle can be used, however latex (blue color) or nanometer sized particles[8] of gold (red color) are most commonly used. The gold particles are red in color due to localized surface plasmon resonance. Fluorescent[9] or magnetic[10][11] labelled particles can also be used, however these require the use of an electronic reader to assess the test result.
Sandwich assays
Sandwich assays are generally used for larger analytes because they tend to have multiple binding sites.[12] As the sample migrates through the assay it first encounters a conjugate, which is an antibody specific to the target analyte labelled with a visual tag, usually colloidal gold. The antibodies bind to the target analyte within the sample and migrate together until they reach the test line. The test line also contains immobilized antibodies specific to the target analyte, which bind to the migrated analyte bound conjugate molecules. The test line then presents a visual change due to the concentrated visual tag, hence confirming the presence of the target molecules. The majority of sandwich assays also have a control line which will appear whether or not the target analyte is present to ensure proper function of the lateral flow pad.
The rapid, low-cost sandwich-based assay is commonly used for home pregnancy tests which detect human chorionic gonadotropin, hCG, in the urine of pregnant women.
Competitive assays
Competitive assays are generally used for smaller analytes since smaller analytes have fewer binding sites[13]. The sample first encounters antibodies to the target analyte labelled with a visual tag (colored particles). The test line contains the target analyte fixed to the surface. When the target analyte is absent from the sample, unbound antibody will bind to these fixed analyte molecules, meaning that a visual marker will show. Conversely, when the target analyte is present in the sample, it binds to the antibodies to prevent them binding to the fixed analyte in the test line, and thus no visual marker shows. This differs from sandwich assays in that no band means the analyte is present.
Quantitative tests
Most tests are intended to operate on a purely qualitative basis. However it is possible to measure the intensity of the test line to determine the quantity of analyte in the sample. Handheld diagnostic devices known as lateral flow readers are used by several companies to provide a fully quantitative assay result. By utilizing unique wavelengths of light for illumination in conjunction with either CMOS or CCD detection technology, a signal rich image can be produced of the actual test lines. Using image processing algorithms specifically designed for a particular test type and medium, line intensities can then be correlated with analyte concentrations. One such handheld lateral flow device platform is made by Detekt Biomedical L.L.C..[14] Alternative non-optical techniques are also able to report quantitative assays results. One such example is a magnetic immunoassay (MIA) in the lateral flow test form also allows for getting a quantified result. Reducing variations in the capillary pumping of the sample fluid is another approach to move from qualitative to quantitative results. Recent work has, for example, demonstrated capillary pumping with a constant flow rate independent from the liquid viscosity and surface energy.[6][15][16][17]
Mobile phones have demonstrated to have a strong potential for the quantification in lateral flow assays, not only by using the camera of the device, but also the light sensor or the energy supplied by the mobile phone battery.[18]
Control line
While not strictly necessary, most tests will incorporate a second line which contains an antibody that picks up free latex/gold in order to confirm the test has operated correctly.[citation needed]
Blood plasma extraction
Because the intense red color of hemoglobin interferes with the readout of colorimetric or optical detection-based diagnostic tests, blood plasma separation is a common first step to increase diagnostic test accuracy. Plasma can be extracted from whole blood via integrated filters[19] or via agglutination[20].
Speed and simplicity
Time to obtain the test result is a key driver for these products. Tests can take as little as a few minutes to develop. Generally there is a trade off between time and sensitivity: more sensitive tests may take longer to develop. The other key advantage of this format of test compared to other immunoassays is the simplicity of the test, by typically requiring little or no sample or reagent preparation.[citation needed]
Patents
This is a highly competitive area and a number of people claim patents in the field, most notably Alere (formerly Inverness Medical Innovations, now owned by Abbott) who own patents[21] originally filed by Unipath. A group of competitors are challenging the validity of the patents.[22] A number of other companies also hold patents in this arena.
Applications
Lateral flow assays have a wide array of applications and can test a variety of samples like urine, blood, saliva, sweat, serum, and other fluids. They are currently used by clinical laboratories, hospitals, and physicians for quick and accurate tests for specific target molecules and gene expression. Other uses for lateral flow assays are food and environmental safety and veterinary medicine for chemicals such as diseases and toxins.[23] Lateral flow tests are also commonly used for disease identification such as ebola, but the most common lateral flow test is the home pregnancy test.
References
- ↑ Concurrent Engineering for Lateral-Flow Diagnostics (IVDT archive, Nov 99)
- ↑ Koczula, Katarzyna M.; Gallotta, Andrea (2016-06-30). "Lateral flow assays". Essays in Biochemistry 60 (1): 111–120. doi:10.1042/EBC20150012. ISSN 0071-1365. PMID 27365041.
- ↑ Yetisen A. K. (2013). "Paper-based microfluidic point-of-care diagnostic devices". Lab on a Chip 13 (12): 2210–2251. doi:10.1039/C3LC50169H. PMID 23652632. https://semanticscholar.org/paper/dbe9e0546e94ccba6732679cfbe080f81e6415ca.
- ↑ "Archived copy". Archived from the original on 2012-07-28. https://web.archive.org/web/20120728025400/http://www.khufash.com/bd/applications/lateral_flow/lf_main_page.htm. Retrieved 2012-07-27.
- ↑ Jonas Hansson; Hiroki Yasuga; Tommy Haraldsson; Wouter van der Wijngaart (2016). "Synthetic microfluidic paper: high surface area and high porosity polymer micropillar arrays". Lab on a Chip 16 (2): 298–304. doi:10.1039/C5LC01318F. PMID 26646057. http://urn.kb.se/resolve?urn=urn:nbn:se:kth:diva-180009.
- ↑ 6.0 6.1 Weijin Guo; Jonas Hansson; Wouter van der Wijngaart (2016). "Viscosity Independent Paper Microfluidic Imbibition". MicroTAS 2016, Dublin, Ireland. https://www.diva-portal.org/smash/get/diva2:1048748/FULLTEXT01.pdf.
- ↑ "Sample Collection & Transport | Sample Preparation | Sample Analysis". http://www.porex.com/products/clinical-sciences/in-vitro-diagnostics/.
- ↑ Quesada-González, Daniel; Merkoçi, Arben (2015). "Nanoparticle-based lateral flow biosensors". Biosensors & Bioelectronics 15 (special): 47–63. doi:10.1016/j.bios.2015.05.050. PMID 26043315. http://ddd.uab.cat/record/200195.
- ↑ (Point-of-Care Technologies) Developing rapid mobile POC systems - Part 1: Devices and applications for lateral-flow immunodiagnostics (IVDT archive, Jul 07)
- ↑ Paramagnetic-particle detection in lateral-flow assays (IVDT archive, Apr 02)
- ↑ "Magnetic immunoassays: A new paradigm in POCT (IVDT archive, Jul/Aug 2008)". http://www.devicelink.com/ivdt/archive/08/07/008.html.
- ↑ nanoComposix. "Introduction to Lateral Flow Rapid Test Diagnostics" (in en). https://nanocomposix.com/pages/introduction-to-lateral-flow-rapid-test-diagnostics.
- ↑ nanoComposix. "Introduction to Lateral Flow Rapid Test Diagnostics" (in en). https://nanocomposix.com/pages/introduction-to-lateral-flow-rapid-test-diagnostics.
- ↑ "Detekt Biomedical L.L.C.- Lateral Flow Readers for Rapid Test Strip Detection and Immunoassays" (in en-US). http://idetekt.com/.
- ↑ Weijin Guo; Jonas Hansson; Wouter van der Wijngaart (2016). "Capillary Pumping Independent of Liquid Sample Viscosity". Langmuir 32 (48): 12650–12655. doi:10.1021/acs.langmuir.6b03488. PMID 27798835.
- ↑ Weijin Guo; Jonas Hansson; Wouter van der Wijngaart (2017). Capillary pumping with a constant flow rate independent of the liquid sample viscosity and surface energy. 339–341. doi:10.1109/MEMSYS.2017.7863410. ISBN 978-1-5090-5078-9. http://urn.kb.se/resolve?urn=urn:nbn:se:kth:diva-196738.
- ↑ Weijin Guo; Jonas Hansson; Wouter van der Wijngaart (2018). "Capillary pumping independent of the liquid surface energy and viscosity". Microsystems & Nanoengineering 4 (1): 2. doi:10.1038/s41378-018-0002-9. PMID 31057892. Bibcode: 2018MicNa...4....2G.
- ↑ Quesada-González, Daniel; Merkoçi, Arben (2017-06-15). "Mobile phone-based biosensing: An emerging "diagnostic and communication" technology" (in en). Biosensors and Bioelectronics 92: 549–562. doi:10.1016/j.bios.2016.10.062. ISSN 0956-5663. PMID 27836593. http://ddd.uab.cat/record/194894.
- ↑ "Passive blood plasma separation at the microscale: a review of design principles and microdevices". J. Micromech. Microeng. 25. 2015. doi:10.1088/0960-1317/25/8/083001. https://doi.org/10.1088/0960-1317/25/8/083001.
- ↑ Guo, Weijin; Hansson, Jonas; van der Wijngaart, Wouter (2020). "Synthetic Paper Separates Plasma from Whole Blood with Low Protein Loss". Analytical Chemistry. doi:10.1021/acs.analchem.0c01474. ISSN 0003-2700.
- ↑ U.S. Patent No. 6,485,982
- ↑ (News) Grassroots Web group challenging lateral-flow patents (IVDT archive, Nov 00)
- ↑ Koczula, Katarzyna M.; Gallotta, Andrea (2016-06-30). "Lateral flow assays". Essays in Biochemistry 60 (1): 111–120. doi:10.1042/EBC20150012. ISSN 0071-1365. PMID 27365041.
 |

