Physics:Mass–action ratio
The mass–action ratio,[1][2] often denoted by [math]\displaystyle{ \Gamma }[/math], is the ratio of the product concentrations, p, to reactant concentrations, s. The concentrations may or may not be at equilibrium.
[math]\displaystyle{ \Gamma = \frac{ p_1 p_2 \ldots}{ s_1 s_2 \ldots} }[/math]
This assumes that the stoichiometric amounts are all unity. If not, then each concentration must be raised to the power of its corresponding stoichiometric amount. If the product and reactant concentrations are at equilibrium then the mass–action ratio will equal the equilibrium constant. At equilibrium:
[math]\displaystyle{ \Gamma = K_{eq} }[/math]
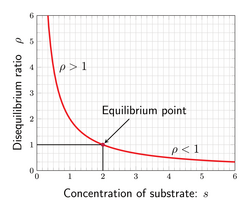
The ratio of the mass–action ratio to the equilibrium constant is often called the disequilibrium ratio, denoted by the symbol [math]\displaystyle{ \rho }[/math].
[math]\displaystyle{ \rho = \frac{\Gamma}{K_{eq}} }[/math]
and is a useful measure for indicating how from equilibrium a given reaction is. At equilibrium [math]\displaystyle{ \rho = 1 }[/math]. The ratio is always greater than zero, When the reaction is out of equilibrium, [math]\displaystyle{ \rho \neq 1 }[/math]. If the reaction has a negative free energy, then [math]\displaystyle{ \rho \lt 1 }[/math].
For a uni-molecular reaction such as [math]\displaystyle{ A \rightleftharpoons B }[/math], where the net reaction rate is given by the reversible mass-action ratio:
[math]\displaystyle{ v = k_1 A - k_2 B = v_f - v_r }[/math]
At thermodynamic equilibrium the rate equals zero, that is [math]\displaystyle{ 0 = k_1 A_{eq} - {k_2 B_{eq}} }[/math]. Rearranging gives:
[math]\displaystyle{ \frac{k_1}{k_2} = \frac{B_{eq}}{A_{eq}} = K_{eq} }[/math]
but [math]\displaystyle{ \rho = \frac{\Gamma}{K_{eq}} }[/math], therefore [math]\displaystyle{ \rho = \Gamma\frac{k_2}{k_1} }[/math] and therefore [math]\displaystyle{ \rho = \frac{B}{A} \frac{k_2}{k_1} = \frac{v_r}{v_f} }[/math]
In other words the disequilibrium ratio is the ratio of the reverse to the forward rate. When the reverse rate, [math]\displaystyle{ v_r }[/math] is less than the forward rate, the ratio is less than one, [math]\displaystyle{ \rho \lt 1 }[/math], indicating that the net reaction is from left to right.
Relationship to Free Energy
If the natural log is taken on both sides of the disequilibrium ratio and both sides is multiplied by RT, one obtains:
[math]\displaystyle{ \ln (\rho)=\ln (\Gamma)-\ln \left(K_{\mathrm{eq}}\right) }[/math]
[math]\displaystyle{ R T \ln (\rho) = -R T \ln K_{e q} + R T \ln \Gamma }[/math]
However, [math]\displaystyle{ -R T \ln K_{e q} }[/math] is the standard free energy, so that [math]\displaystyle{ R T \ln (\rho) }[/math] is the free energy of the reaction.
This shows that the free energy of a reaction is just an alternative way of expressing the disequilibrium ratio and as such gives a more intuitive interpretation of free energy. That is if the free energy for a reaction is less than zero then it indicates that [math]\displaystyle{ \rho \lt 1 }[/math] and hence [math]\displaystyle{ v_r \lt v_f }[/math], i.e the net reaction rate is from left to right.
References
- ↑ B. Hess and K. Brand. (1965). Enzymes and metabolite profiles. In Control of energy metabolism. III. Ed. B. Chance, R. K. Estabrook and J. R. Williamson. New York: Academic Press.
- ↑ Haynie, Donald T. (2001). Biological thermodynamics. Cambridge: Cambridge University Press. ISBN 9780511754784.
Other sources
- Atkins, P.W. (1978). Physical Chemistry Oxford University Press ISBN:0-7167-3539-3
- Trevor Palmer (2001) Enzymes: biochemistry, biotechnology and clinical chemistry Chichester Horwood Publishing ISBN:1-898563-78-0
 |