Physics:Metastable inner-shell molecular state


Metastable Innershell Molecular State (MIMS)[1][2][3][4] is a class of ultra-high-energy short-lived molecules have the binding energy up to 1,000 times larger and bond length up to 100 times smaller than typical molecules. MIMS is formed by inner-shell electrons that are normally resistant to molecular formation. However, in stellar conditions, the inner-shell electrons become reactive to form molecular structures (MIMS) from combinations of all elements in the periodic table.
MIMS upon dissociation can emit x-ray photons with energies up to 100 keV at extremely high conversion efficiencies from compression energy to photon energy. MIMS is predicted to exist and dominate radiation processes in extreme astrophysical environments, such as large planet cores, star interiors, and black hole and neutron star surroundings. There, MIMS is predicted to enable highly energy-efficient transformation of the stellar compression energy into the radiation energy.
The right schematic illustration shows the proposed four stages of the K-shell MIMS (K-MIMS) formation and x-ray generation process. Stage I: Individual atoms are subjected to the stellar compression and ready for absorbing the compression energy. Stage II: The outer electron shells fuse together under increasing "stellar" pressure. Stage III: At the peak pressure, via pressure ionization K-shell orbits form the K-MIMS, which is vibrationally hot and encapsulated by a Rydberg-like pseudo-L-Shell structure. Stage IV: The K-MIMS cools down by ionizing ("boiling-off") a number of pseudo-L-shell electrons and subsequent optical decay by emitting an x-ray photon. The dissociated atoms return their original atoms states and are ready for absorbing the compression energy.
MIMS also can be readily produced in laboratory and industrial environments, such as hypervelocity particle impact, laser fusion and z-machine. MIMS can be exploited for highly energy-efficient production of high intensity x-ray beams for a wide range of innovative applications, such as photolithography, x-ray lasers, and inertial fusion.
History
The inner-shell-bound metastable quasimolecules were proposed to exist in the x-ray generating scattering process since the work by Mott in the 1930s.[5] The existence of high energy quasimolecules in highly compressed matter (or strongly coupled plasma) was theoretically predicted in the ab initio quantum calculation by Younger et al. in the late 1980s.[6] In 2008, from the result obtained by Mueller, Rafelski and Greiner[7] for quasimolecules in atomic collisions at high impact velocity, Winterberg[8] predicted the existence of inner-shell bound metastable keV molecules under high pressure and their use for the ignition of thermonuclear reactions. Metastable Innsershell Molecular State (MIMS) that is homologous to the rare gas excimers was proposed by Bae in 2008[1] to interpret the mysterious anomalous x-ray signals observed by Bae and his colleagues at Brookhaven National Lab (BNL) in the 1990s.[9][10] For more details, refer to the last section of this article, "Other models for inner-shell-bound molecules."
To search for many-body effects in the highly compressed stellar materials, Bae and his colleagues at BNL generated and studied such materials by impacting various bio and water nanoparticle at hypervelocities (v~100 km/s) on various targets.[9][10] In their study, anomalous signals were discovered, when the nanoparticles were directly impacted on and detected by Si particle detectors that had windows sufficiently thick enough to block the penetration of the nanoparticles completely. By exploiting the discovered anomalous signals, the feasibility of generating highly compressed "stellar" matter at shock pressures on the order of 10 TPa (100 Mbar) with the nanoparticle impact in a non-destructive laboratory setup was proven. However, the nature of the signals and the underlying physics of their generation mechanism had not been understood for 15 years.
It was not until 2008 that Bae was able to unlock the mystery of the anomalous BNL signals owing to emerging sciences of the stellar materials.[8][11][12] In the analysis of the BNL signals, Bae discovered[1] that a new class of ultra-high-energy metastable molecules that are bound by inner-shell electrons was responsible for the signals and named the molecules Metastable Innershell Molecular State (MIMS). Further, Bae discovered that the observed energy conversion efficiency via MIMS from the nanoparticle kinetic energy to the radiation energy was as high as 40%, thus proposed that MIMS can enable a new generation of ultra-high efficiency compact x-ray generators.
In 2012, Bae independently confirmed[13] the BNL results with buckyball ions (C60+) impacting on an Al target in an independent tabletop apparatus that is orders of magnitude more compact than that at BNL. The result also demonstrated the potential of scaling up of x-ray generation with nanoparticle impact by exploiting C60+ ions, of which currents can be readily scaled up to an industrial quantity in a tabletop apparatus. Bae also proposed a more elaborated MIMS model that is homologous to rare gas excimer molecules was developed and predicted that all elements in periodic table are subjected to the MIMS formation.[13] Up until then, the observed MIMS was proposed to be formed with L-shell electrons.
In MIMS researches that involve a wide range of x-ray generation phenomena in 2013–2014, Bae discovered[2][3] that the manifestations of MIMS that was formed with K-shell electrons have existed in extensive experimental data in the x-ray generating heavy ion collision process by numerous researchers for several decades.[14][15][16] In his papers,[2][3] Bae proposed that the quasimolecule is a manifestation of MIMS during the collisional process, a special circumstance for producing MIMS. After extensive analyses and theoretical modeling of these data, which involve a wide range of elements in the periodic table, a successful integration of the data into the frame of the unified MIMS model was demonstrated. Thereby, the MIMS model was firmly established and confirmed for any combinations of all elements in the periodic table.
Specifically, the extensive analyses[2][3] of the data that relate to hard x-ray generating collisions have resulted in a universal law (Z2–dependency) of the binding energy of the homonucleus MIMS bound by K-shell electrons (K-MIMS). Here Z is the atomic number of the constituent atoms of the K-MIMS. Bae further developed a unified theory to elucidate the Z2-dependent behavior of the homonucleus K-MIMS, which behaves much like the helium excimer molecule: He2*.[2][3] The MIMS theory also predicted a 1/Z dependency law for the bond length of the homonucleus K-MIMS. Based on the MIMS theory the uranium K-MIMS, for example, is predicted to have a 100 times smaller bond length, a 2,000 times larger binding energy, and a 5,000 times larger characteristic x-ray energy than the He excimer molecule.[17] The predicted bond lengths of the bismuth and uranium K-MIMS are in excellent agreement with that estimated from the experimental results by researchers at the GSI Helmholtz Centre for Heavy Ion Research in Darmstadt, Germany [18]
MIMS Model

In typical environment, the ground state of rare gas atom dimers is electronically non-binding, but if their closed outershell electrons are excited, dimers can readily form transient bound molecules, excimers. For example, the ground He2 state (1sσg21sσu2: X1Σg+) is electronically repulsive, but excitation of an electron can lead to Rydberg states (for example, the metastable, 1sσg21sσu2sσg: a3Σu+) with the He2+core.[20] The low-lying metastable excited states of the dimer (the He2 excimer) are strongly covalently bound. The metastable excimer can radiate to the free repulsive ground state.
The excimer formation is a critical step in energy-efficient conversion of the atomic electron excitation/ionization energy into the radiation energy in rare gas plasma for the excimer lasers. Without forming the excimers, the energy of the excited atoms would be rapidly lost by non-radiative collisional decay processes in the high pressure environment. In other words, the excimer formation is a crucial step needed for efficient transformation of the atomic excitation energy to the radiation energy in an ultra-high pressure environment. Analogously, the MIMS formation is a crucial step required for efficiently transforming the atomic core-excitation energy into the radiation energy.[2][3]
Based on the MIMS model,[1][2][3] a schematic potential curve was proposed for the K-shell MIMS (K-MIMS) and illustrated in the right figure. In the "electronically-cold" highly compressed plasma, a K-shell core ion with a hole, [K1], collides with another core ion without a hole, [K0], to form a near-dissociative K-shell MIMS (K-MIMS) and its excess energy transforms into the vibration energy. Such a vibrationally hot K-MIMS in the plasma is denoted here by K1 + K0. Subsequently, a Rydberg-like pseudo-L-shell forms around the K-MIMS core ions that are proposed to go through a rapid relaxation into the lowest vibrational state by ionizing a number of electrons in the pseudo-L-shell.[2][3] The vibrationally cold K-MIMS without an L-shell hole is denoted by [K1K0L0]. The vibrational energy is quenched by ionizing the bound [K1K0L0] MIMS into a higher ionic state [K1K0Ln] with n holes in the pseudo-L-shell, where 1 ≤ n ≤ 8. Because the n holes can be distributed among 2s and 2p orbits, thus the maximum value of n is 8.[21] The statistically distributed K-MIMS decays into the lower L-shell MIMS (L-MIMS), [K0K0L1], by emitting an x-ray photon, and subsequently the L-MIMS dissociates into two atomic ions.[2][3]
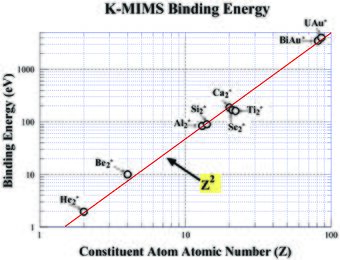
Based on the previously proposed MIMS model,[1][2][3] a wide range of the K-MIMS binding energies were extracted from the extensive ranges of experimental data from He2* excimer to UAu* MIMS (for 2 ≤ Z ≤ 92). The right shows these data plotted against the constituent atom atomic number Z. The lowest bound state (a3Σu+) of He2* excimer has a binding energy of 1.96 eV, which is plotted in the right figure. These data are plotted against Z in the figure.
The overall data trend shows a Z2 dependency. Overall fitting is excellent, thus indicates that the K-MIMS can be modeled with a He2*-like excimer with core K-shell ions with an atomic number Z to the first order. Note that the binding energies are about one order of magnitude smaller than the corresponding K-shell satellite x-ray energies, which follow the (Z-1)2 dependency of the Moseley's law.

An intuitive analytical theory has been developed and is presented here to illuminate the universal Z2-dependent behavior of K-MIMS by Bae. In this theory, the homologous molecular characteristics of K-MIMS is proposed to be primarily determined by the 1sσg2 bonding molecular orbit of the homologous core molecular ion under the assumption that other effects of surrounding electrons and atoms can be considered as a minor perturbation. The quantum characteristics of excimer can be described by a stable core molecular ion surrounded by Rydberg-like electron orbits. The lowest metastable electronic state of He2* (a3Σu+) is 1sσg21sσu2sσg, thus the proposed K-MIMS model should have a similar molecular orbital. In this case the bonding of K-MIMS is expected to be primarily dominated by the 1sσg2 orbital. Intuitively, the 1sσg2 orbital can be approximated in the frame of the LCAO model by a linear combination of the two 1s atomic orbitals, of which size is proportional to 1/Z. Therefore, the K-MIMS size, thus the bond length, is predicted to be proportional to 1/Z. Therefore, to the first order the K-MIMS bond length is predicted to be proportional to 1/Z. The right figure shows the predicted K-MIMS bond length as a function of the constituent atom atomic number, Z. The bond length of He2* (a3Σu+) is ~1.05 A, and the solid line represents a 1/Z curve that is extrapolated from the bond length of He2* (a3Σu+).
Currently, there is no other direct experimental data or theoretical results on the bond length of the K-MIMS. However, in the recent work by Mokler's group,[18] the sizes of quasimolecules were estimated from the x-ray cross-section enhancement of both projectile x-rays and target x-rays in H-like ion impact. The enhancement was interpreted to result from an extensive K-K electron sharing (transition of a hole) between the projectile and target ions due to the quasimolecule effect during the collision. Based on the K-MIMS theory as illustrated in the right figure, the K-K sharing distance can approximate the K-MIMS bond length. The two data points are the K-K sharing distances of U91+ ion impact on Au and Bi82+ ion impact on Au, which were estimated from the cross sections. The Z values were approximated by the (Zp +Zt)/2, where Zp is the atomic number of the projectile ion and Zt is that of the target atom. The predicted 1/Z curve is in excellent agreement with these data points as shown in the right figure.
MIMS can be also formed with two different elements.[4] Currently, such heteronucleus MIMS formed with H+ and He+ with other elements are proposed to be observed in H+ and He+ impact on a range of solids. Based on Equation of States (EOS) of materials,[11][12] it can be predicted that pressures required to form homonucleus L-shell MIMS are on the order of 100 Mbar (10 TPa), while homonucleus K-shell MIMS on the order of 10 – 100 Gbar (1,000 – 10,000 TPa). Pressures required to form heteronucleus MIMS are predicted to be considerably smaller than that for homonucleus MIMS.
Other models for inner-shell bound molecules
Quasimolecules in heavy ion collisions
In the field of x-ray generation by heavy ion impact on solids, the search for the x-ray signatures of the inner-shell-bound quasimolecule in the x-ray generating scattering process can be traced down to the work by Mott in the 1930s.[5] The quasimolecule can be considered as a collisional complex that is a manifestation of MIMS during collision processes. However, the actual experimental searches for such x-ray signatures of the quasimolecule in x-ray generation in heavy ion impact started much later, in the 1970s.[16] One of the primary motivations of these researches was to explore a super-heavy quasimolecule/quasiatom with a combined atomic number exceeding 100, which was predicted to behave like a transuranium atom in the united atom frame.
In the early heavy ion collision researches, the cross-sections for carbon K x-ray production were measured for a wide range of ions incident on a carbon target, at energies from 20 to 80 keV.[22] The carbon Kα x-ray generation cross sections for the heavy ions, such as Ar+ and Xe+, were discovered to be several orders of magnitude larger than those by light ions, such as H+ and He+, which were consistent with the values predicted by the direct Coulomb scattering theory.[16] The anomalous cross sections for heavy ions were qualitatively interpreted in terms of the electron-promotion mechanism of the molecular orbital theory.[23] In his papers, Bae proposed that the production of the shocked regions that are able to bear abundant MIMS by the heavy ion impacts as in the nanoparticle impact[1][9][10][13] can be another major factor for the observed x-ray yield enhancement.
More importantly, there had been extensive theoretical and experimental researches on intense K-shell satellites above the Kα line of various solids that were bombarded by heavy ions with kinetic energies on the order of 10 MeV.[14] In the researches, the K-shell satellites were interpreted to result from radiative decays of atoms with multiple holes, one of which is in the K-shell and others in the L-shell. Striking differences between the satellite spectra obtained with light ions, such as electrons and protons, and the spectra with heavy ions were discovered. In addition to the intensity difference, the x-ray spectra obtained with heavy ions showed many more peaks with multiple L-shell holes than those with light ions. The x-ray energies of the satellites were consistent with those obtained by the ab initio calculations based on the atomic model x-ray emissions that involve varying number of L-shell holes. However, the satellite data for larger number of L-shell holes significantly deviated from the calculation results. Based on the MIMS model, the x-ray satellite structures are now understood to result from the interaction between the vibrational state of K-MIMS and the L-shell electron ionization channels.[2][19]
Younger's quasimolecules
Overall, Younger et al.[6] demonstrated the possibility of forming transient molecular states with closed-shell electrons in ab initio calculations for the first time for frozen or slow moving ion systems that can be approximated with the Born-Oppenheimer approximation.
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 Y.K. Bae (2008). "Metastable inner-shell molecular state (MIMS)". Physics Letters A 372 (29): 4865–4869. doi:10.1016/j.physleta.2008.05.037. Bibcode: 2008PhLA..372.4865B. http://www.sciencedirect.com/science/article/pii/S037596010800741X.
- ↑ 2.00 2.01 2.02 2.03 2.04 2.05 2.06 2.07 2.08 2.09 2.10 Y.K. Bae (2014). "Metastable Innershell Molecular State (MIMS) II: K-shell X-ray satellites in heavy ion impact on solids". Results in Physics 4: 170–176. doi:10.1016/j.rinp.2014.09.005. Bibcode: 2014ResPh...4..170B. http://www.sciencedirect.com/science/article/pii/S2211379714000515.
- ↑ 3.00 3.01 3.02 3.03 3.04 3.05 3.06 3.07 3.08 3.09 Bae, Young K. (2016-03-11). "Metastable innershell molecular state (MIMS) III: The universal binding energy and bond length of the homonucleus K-shell MIMS". Physics Letters A 380 (11–12): 1178–1183. doi:10.1016/j.physleta.2014.09.024. Bibcode: 2016PhLA..380.1178B. http://www.sciencedirect.com/science/article/pii/S0375960114009256.
- ↑ 4.0 4.1 Y.K. Bae (2014). "Metastable innershell molecular state (MIMS) IV: Heteronucleus K-shell MIMS with H+ and He+". Results in Physics 4: 198–203. doi:10.1016/j.rinp.2014.10.002. Bibcode: 2014ResPh...4..198B. http://www.sciencedirect.com/science/article/pii/S2211379714000539.
- ↑ 5.0 5.1 Mott, N.F. (1931). "On the Theory of Excitation by Collision with Heavy Particles". Proceedings of the Cambridge Philosophical Society 27 (4): 553. doi:10.1017/s0305004100009816. Bibcode: 1931PCPS...27..553M.
- ↑ 6.0 6.1 Younger, S.M. (1988). "Quantum calculations of the electronic structures of a many-atom plasma" (Submitted manuscript). Physical Review Letters 61 (8): 962–965. doi:10.1103/physrevlett.61.962. PMID 10039479. Bibcode: 1988PhRvL..61..962Y. https://zenodo.org/record/1233866.
- ↑ B. Mueller; J. Rafelski; W. Greiner (1973). "Solution of the Dirac equation with two Coulomb centres". Physics Letters 47B (1): 5. doi:10.1016/0370-2693(73)90554-6. Bibcode: 1973PhLB...47....5M.
- ↑ 8.0 8.1 F. Winterberg (2008). "Transient Formation of Super-Explosives under High Pressure for Fast Ignition". Zeitschrift für Naturforschung 63a: 35. doi:10.1515/zna-2008-1-206. Bibcode: 2008ZNatA..63...35W.
- ↑ 9.0 9.1 9.2 Y.K. Bae; Y.Y. Chu; L. Friedman (1995). "Observation of enhancement of stopping power and possible hydrodynamic shock behavior in penetration of large molecules in solids". Physical Review A A51 (3): R1742. doi:10.1103/PhysRevA.51.R1742. Bibcode: 1995PhRvA..51.1742B.
- ↑ 10.0 10.1 10.2 Y.K. Bae (1996). "Detection of accelerated large water cluster ions and electrosprayed biomolecules with passivated solid state detectors". Nuclear Instruments and Methods in Physics Research Section B 114 (1): 185–190. doi:10.1016/0168-583x(96)00043-2. Bibcode: 1996NIMPB.114..185B. http://www.sciencedirect.com/science/article/pii/0168583X96000432.
- ↑ 11.0 11.1 B.F. Rozsnyai (2001). "Theory and experiment for ultrahigh pressure shock Hugoniots" (Submitted manuscript). Physics Letters A 291 (4): 226–231. doi:10.1016/s0375-9601(01)00661-2. Bibcode: 2001PhLA..291..226R. https://zenodo.org/record/1260031.
- ↑ 12.0 12.1 J.C. Pain (2007). "Equation-of-state model for shock compression of hot dense matter". Physics Letters A 362 (2–3): 120–124. doi:10.1016/j.physleta.2006.10.013. Bibcode: 2007PhLA..362..120P.
- ↑ 13.0 13.1 13.2 Y.K. Bae (2013). "Creating nanostars with buckball". Physics Letters A 377 (45–48): 3304–3311. doi:10.1016/j.physleta.2013.10.036. Bibcode: 2013PhLA..377.3304B. http://www.sciencedirect.com/science/article/pii/S0375960113009894.
- ↑ 14.0 14.1 Williams 1976, pp. 166–191
- ↑ Crasemann 1975
- ↑ 16.0 16.1 16.2 R. Anholt (1985). "X-rays from quasimolecules". Reviews of Modern Physics 57 (4): 995–1053. doi:10.1103/revmodphys.57.995. Bibcode: 1985RvMP...57..995A.
- ↑ Huber 1979
- ↑ 18.0 18.1 P. Verma (2006). "Probing superheavy quasimolecular collisions with incoming inner shell vacancies". Nuclear Instruments and Methods in Physics Research Section B 245 (1): 56–60. doi:10.1016/j.nimb.2005.11.161. Bibcode: 2006NIMPB.245...56V.
- ↑ 19.0 19.1 19.2 19.3 Y.K. Bae (2014). "Metastable innershell molecular state (MIMS) III: The universal binding energy and bond length of the homonucleus K-shell MIMS". Physics Letters A in press (11–12): 1178–1183. doi:10.1016/j.physleta.2014.09.024. Bibcode: 2016PhLA..380.1178B. http://www.sciencedirect.com/science/article/pii/S0375960114009256.
- ↑ Birks, J.B. (1975). "Excimers". Reports on Progress in Physics (38): 903.
- ↑ Pratt, S.T. (2005). "Vibrational autoionization in polyatomic molecules". Annual Review of Physical Chemistry 56: 281–308. doi:10.1146/annurev.physchem.56.092503.141204. PMID 15796702. Bibcode: 2005ARPC...56..281P. https://zenodo.org/record/1235049.
- ↑ Der, R.C. (1968). "Production of carbon characteristic x-rays by heavy-ion bombardment". Physical Review Letters 21 (26): 1731–1732. doi:10.1103/physrevlett.21.1731. Bibcode: 1968PhRvL..21.1731D.
- ↑ Fano, U.; Lichten, W. (1965). "Interpretation of Ar+ - Ar collisions". Physical Review Letters 14 (16): 627–629. doi:10.1103/physrevlett.14.627. Bibcode: 1965PhRvL..14..627F.
Further reading
- University textbooks and monographs
- Williams, D. (1976). Method of Experimental Physics. Academic Press Press. ISBN 978-0-12-475913-8.
- Crasemann, B. (1975). Atomic Inner-Shell Processes. Academic Press Press. ISBN 978-0-12-196901-1.
- Huber, K.; Herzberg, G. (1979). Molecular Spectra and Molecular Structure IV. Constants of Diatomic Molecules. Van Nostrand Reinhold. ISBN 978-0-442-23394-5.
External links
